Question: Temperature (C) 1. For a binary R-G phase diagram shown below, (a) Determine what type of phase diagram it is, identify and write out
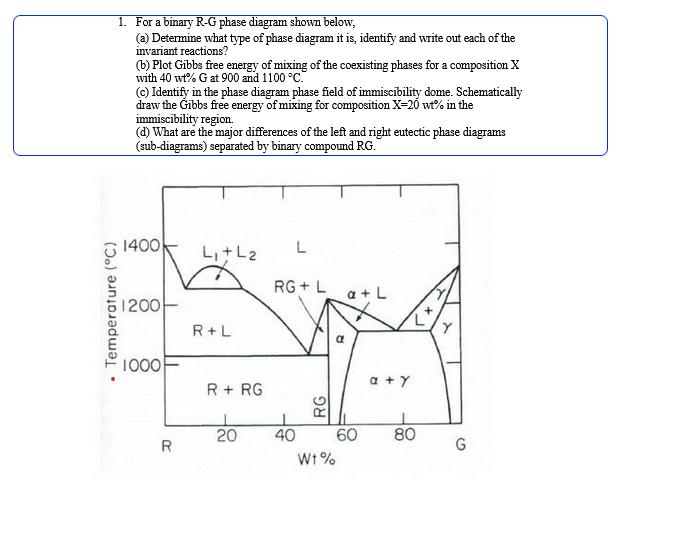
Temperature (C) 1. For a binary R-G phase diagram shown below, (a) Determine what type of phase diagram it is, identify and write out each of the invariant reactions? (b) Plot Gibbs free energy of mixing of the coexisting phases for a composition X with 40 wt% G at 900 and 1100 C. (c) Identify in the phase diagram phase field of immiscibility dome. Schematically draw the Gibbs free energy of mixing for composition X=20 wt% in the immiscibility region. (d) What are the major differences of the left and right eutectic phase diagrams (sub-diagrams) separated by binary compound RG. 1400 L+L L RG+L a+L 1200- R+L 1000- a+Y R + RG RG 20 40 60 80 R G W1%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


