Question: Temperature (C) Phase diagram. Use wt% and Centigrade. (8 pts) (a) What is the maximum solubility of alumina in magnesia at 1900C? (b) What
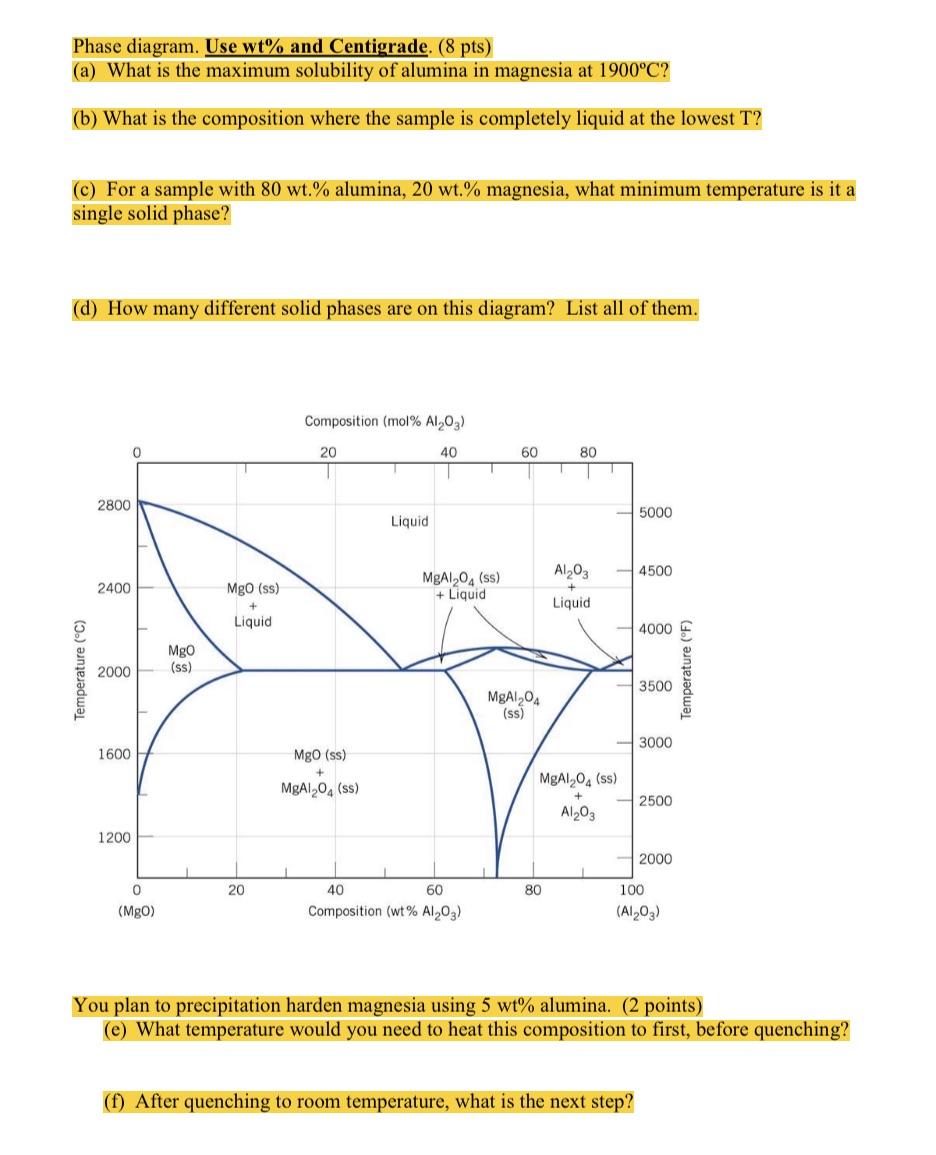
Temperature (C) Phase diagram. Use wt% and Centigrade. (8 pts) (a) What is the maximum solubility of alumina in magnesia at 1900C? (b) What is the composition where the sample is completely liquid at the lowest T? (c) For a sample with 80 wt.% alumina, 20 wt.% magnesia, what minimum temperature is it a single solid phase? (d) How many different solid phases are on this diagram? List all of them. 2800 2400 Composition (mol% Al2O3) 0 20 MgO MgO (ss) + Liquid 2000 (ss) Liquid 40 60 80 5000 MgAl2O (ss) +Liquid Al2O3 -4500 Liquid 4000 MgAl204 (ss) T 3500 3000 1600 MgO (ss) + MgAl204 (ss) MgAl204 (ss) Al2O3 2500 1200 2000 0 (MgO) 20 40 60 80 100 Composition (wt % Al2O3) (Al2O3) Temperature (F) You plan to precipitation harden magnesia using 5 wt% alumina. (2 points) (e) What temperature would you need to heat this composition to first, before quenching? (f) After quenching to room temperature, what is the next step?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


