Question: Tersity VEE200 HW 10 CHEM 1212 (Sections D, E and J) - Spring21 - HURST > Activities and Due Dates > HW 10 ore: 50%
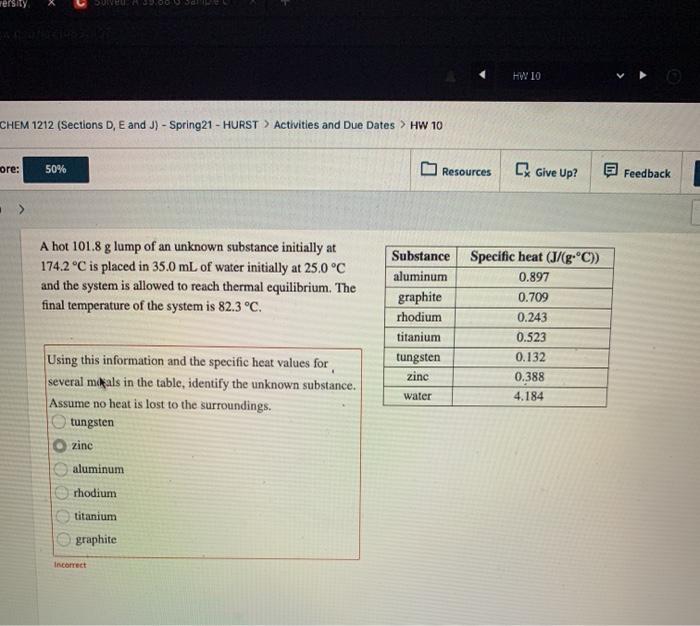
Tersity VEE200 HW 10 CHEM 1212 (Sections D, E and J) - Spring21 - HURST > Activities and Due Dates > HW 10 ore: 50% Resources Give Up? Feedback A hot 101.8 g lump of an unknown substance initially at 174.2C is placed in 35.0 mL of water initially at 25.0 C and the system is allowed to reach thermal equilibrium. The final temperature of the system is 82.3 C. Substance aluminum graphite rhodium titanium tungsten zinc Specific heat (J/(gC)) 0.897 0.709 0.243 0.523 0.132 0.388 4.184 water Using this information and the specific heat values for several mals in the table, identify the unknown substance. Assume no heat is lost to the surroundings. tungsten O zinc aluminum rhodium titanium graphite Incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


