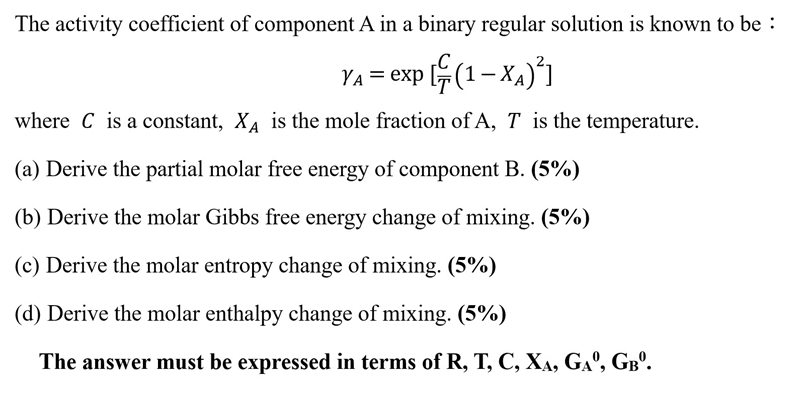
Question: The activity coefficient of component A in a binary regular solution is known to be : A = exp [ C T ( 1 -
The activity coefficient of component in a binary regular solution is known to be :
exp
where is a constant, is the mole fraction of is the temperature.
a Derive the partial molar free energy of component B
b Derive the molar Gibbs free energy change of mixing.
c Derive the molar entropy change of mixing.
d Derive the molar enthalpy change of mixing.
The answer must be expressed in terms of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


