Question: The answer can be short. In addition, please answer all questions, and do not copy other people's answers to me , I will give you
The answer can be short. In addition, please answer all questions, and do not copy other people's answers to me, I will give you a good evaluation based on the answer.
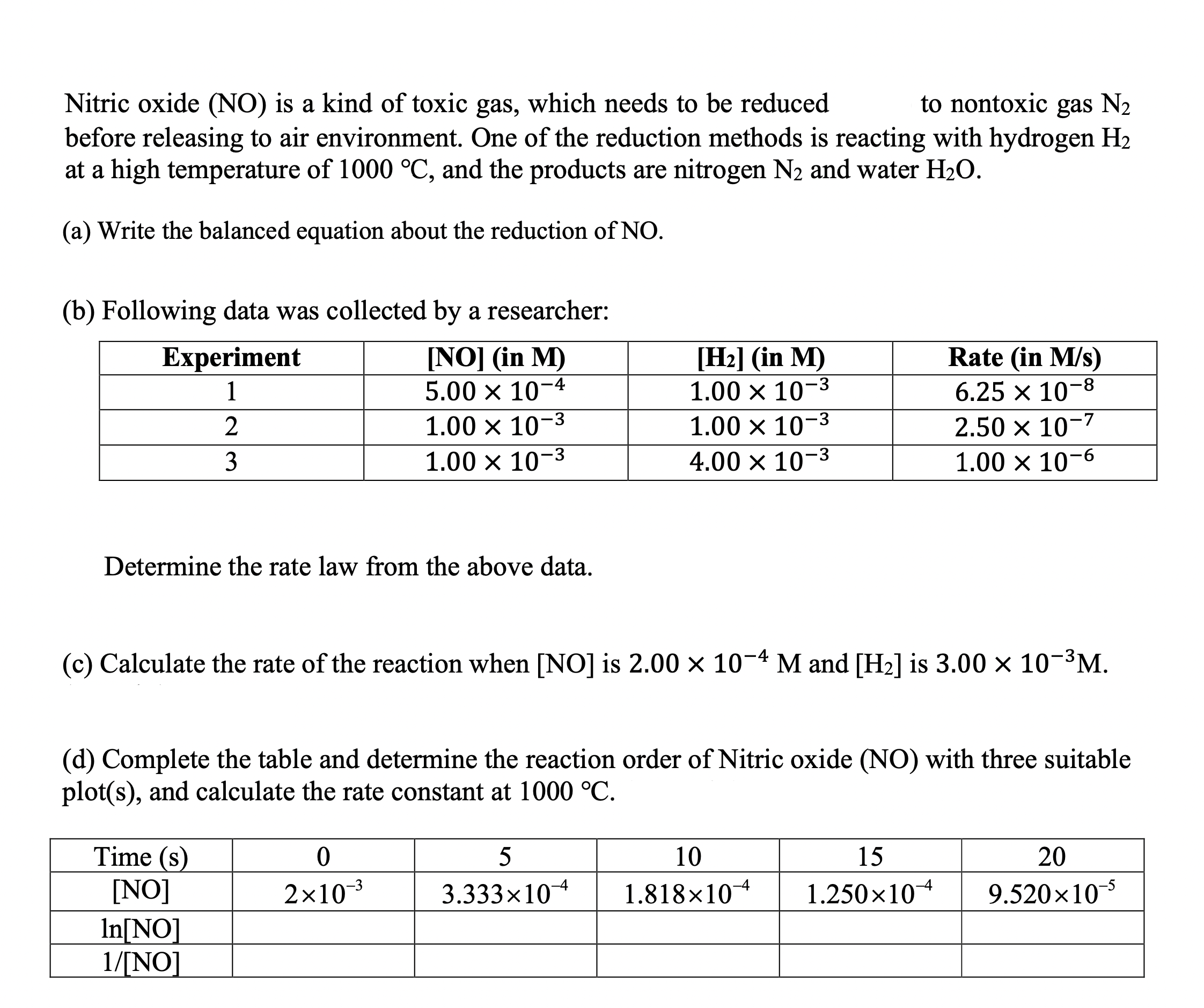
Nitric oxide (NO) is a kind of toxic gas, which needs to be reduced to nontoxic gas N2 before releasing to air environment. One of the reduction methods is reacting with hydrogen H2 at a high temperature of 1000C, and the products are nitrogen N2 and water H2O. (a) Write the balanced equation about the reduction of NO. (b) Following data was collected by a researcher: Determine the rate law from the above data. (c) Calculate the rate of the reaction when [NO] is 2.00104M and [H2] is 3.00103M. (d) Complete the table and determine the reaction order of Nitric oxide (NO) with three suitable plot(s), and calculate the rate constant at 1000C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


