Question: The answer is shown at the bottom part. Please use Joules as unit for the answer. Thank you! 2. 1 mol of CCI, gas has
The answer is shown at the bottom part. Please use Joules as unit for the answer. Thank you!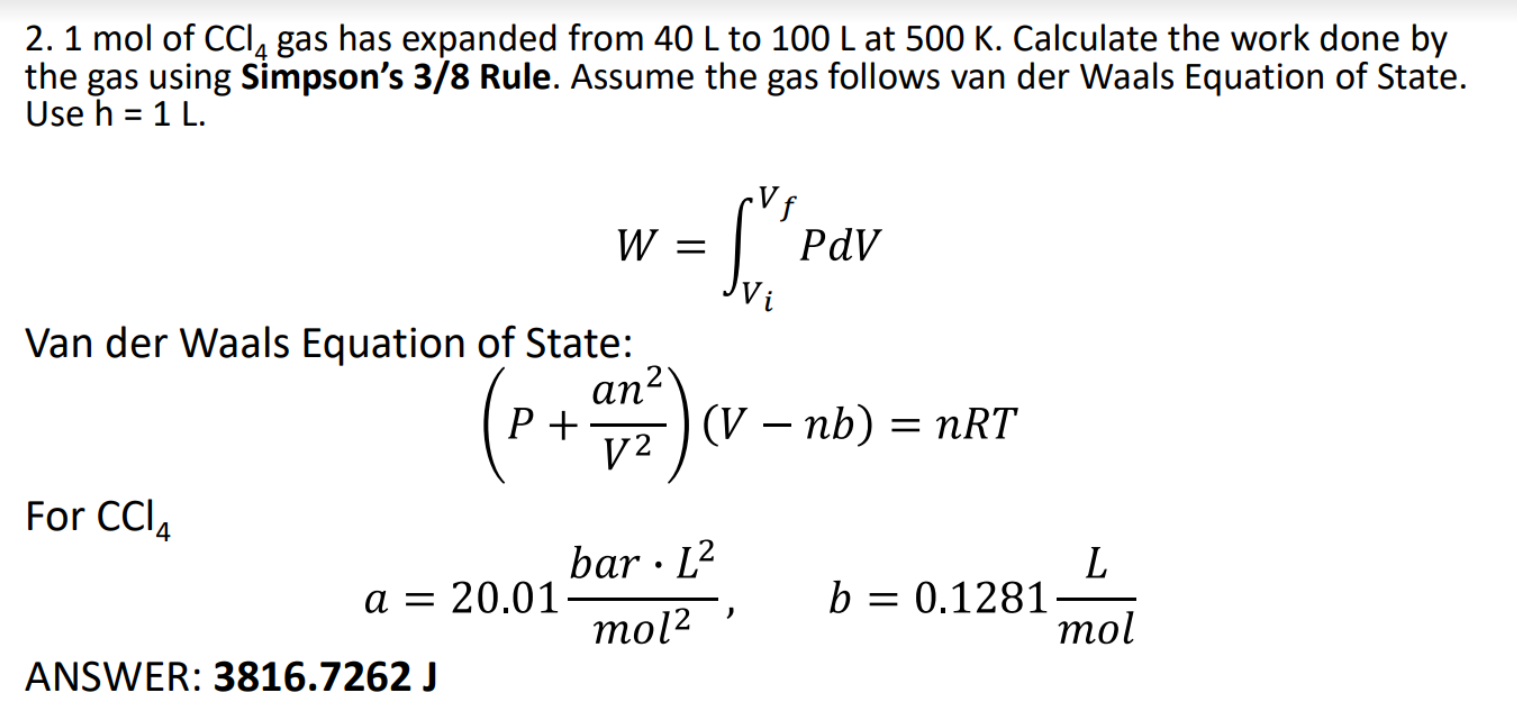
2. 1 mol of CCI, gas has expanded from 40 L to 100 L at 500 K. Calculate the work done by the gas using Simpson's 3/8 Rule. Assume the gas follows van der Waals Equation of State. Use h = 1 L. W = PdV Vi Van der Waals Equation of State: EMP. ) (V nb P + an2 12 (V nb) = nRT For CCIA bar L2 a = 20.01 mol2 ANSWER: 3816.7262 J L b = 0.1281 mol = )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


