Question: The answer key was just passed out. I don't know how these answers were received. We are using Elements of Chemical Reaction Engineering Fifth edition
The answer key was just passed out. I don't know how these answers were received. We are using Elements of Chemical Reaction Engineering Fifth edition by Fogler. Can you explain how these answers were produced? And explain it like I'm an dingdong. Label your equations with things like "This is the design equation" "I built this equation by combining this and that equation" etc. I received a 17% on the test and I'm trying to learn but the book is difficult.
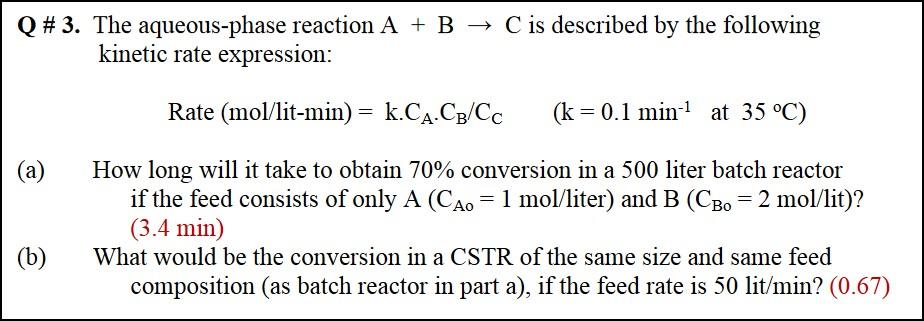
Q # 3. The aqueous-phase reaction A + B + C is described by the following kinetic rate expression: Rate (mol/lit-min) = k.CA.Cg/Cc (k = 0.1 min-1 at 35 C) (a) How long will it take to obtain 70% conversion in a 500 liter batch reactor if the feed consists of only A (CA. = 1 mol/liter) and B (CBo = 2 mol/lit)? (3.4 min) What would be the conversion in a CSTR of the same size and same feed composition (as batch reactor in part a), if the feed rate is 50 lit/ min? (0.67) (b)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


