Question: The answer key was just passed out. I don't know how these answers were received. We are using Elements of Chemical Reaction Engineering Fifth edition
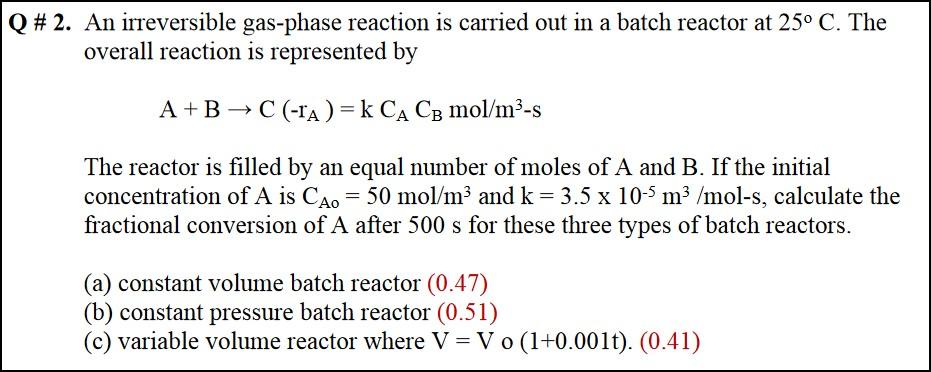
The answer key was just passed out. I don't know how these answers were received. We are using Elements of Chemical Reaction Engineering Fifth edition by Fogler. Can you explain how these answers were produced? And explain it like I'm an dingdong. Label your equations with things like "This is the design equation" "I built this equation by combining this and that equation" etc. I received a 17% on the test and I'm trying to learn but the book is difficult.
Q # 2. An irreversible gas-phase reaction is carried out in a batch reactor at 25 C. The overall reaction is represented by A+B+C (-1A)= k CA CB mol/m3-s The reactor is filled by an equal number of moles of A and B. If the initial concentration of A is CA0 = 50 mol/m and k = 3.5 x 10-5 m3 /mol-s, calculate the fractional conversion of A after 500 s for these three types of batch reactors. (a) constant volume batch reactor (0.47) (b) constant pressure batch reactor (0.51) (c) variable volume reactor where V = V 0 (1+0.001t). (0.41)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


