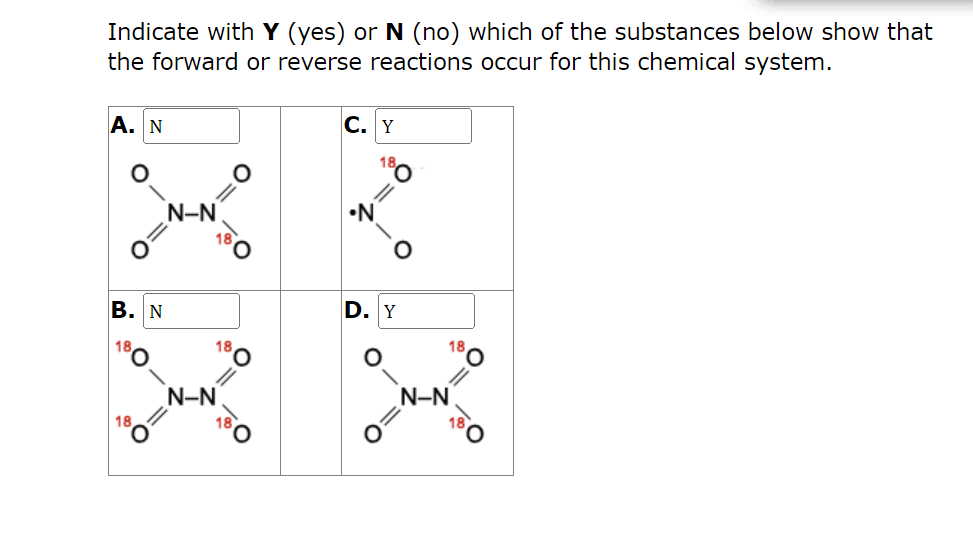
Question: The answers I put are wrong. Please answer all Dinitrogen tetraoxide is a colorless gas that dissociates into nitrogen dioxide, a reddish brown gas. N2O4(g)2NO2(g)

 The answers I put are wrong. Please answer all
The answers I put are wrong. Please answer all
Dinitrogen tetraoxide is a colorless gas that dissociates into nitrogen dioxide, a reddish brown gas. N2O4(g)2NO2(g) An experiment was run to demonstrate that this is a dynamic equilibrium. Starting with a special form of N2O4 where one of the oxygens was isotopically labeled (18O instead of 16O), the system was then allowed to reach equilibrium. Indicate with Y (yes) or N (no) which of the substances below show that the forward or reverse reactions occur for this chemical system
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


