Question: The average molecular mass for ? 1 3 7 C s is 1 3 6 . 9 0 7 g m o l . Avogadro's
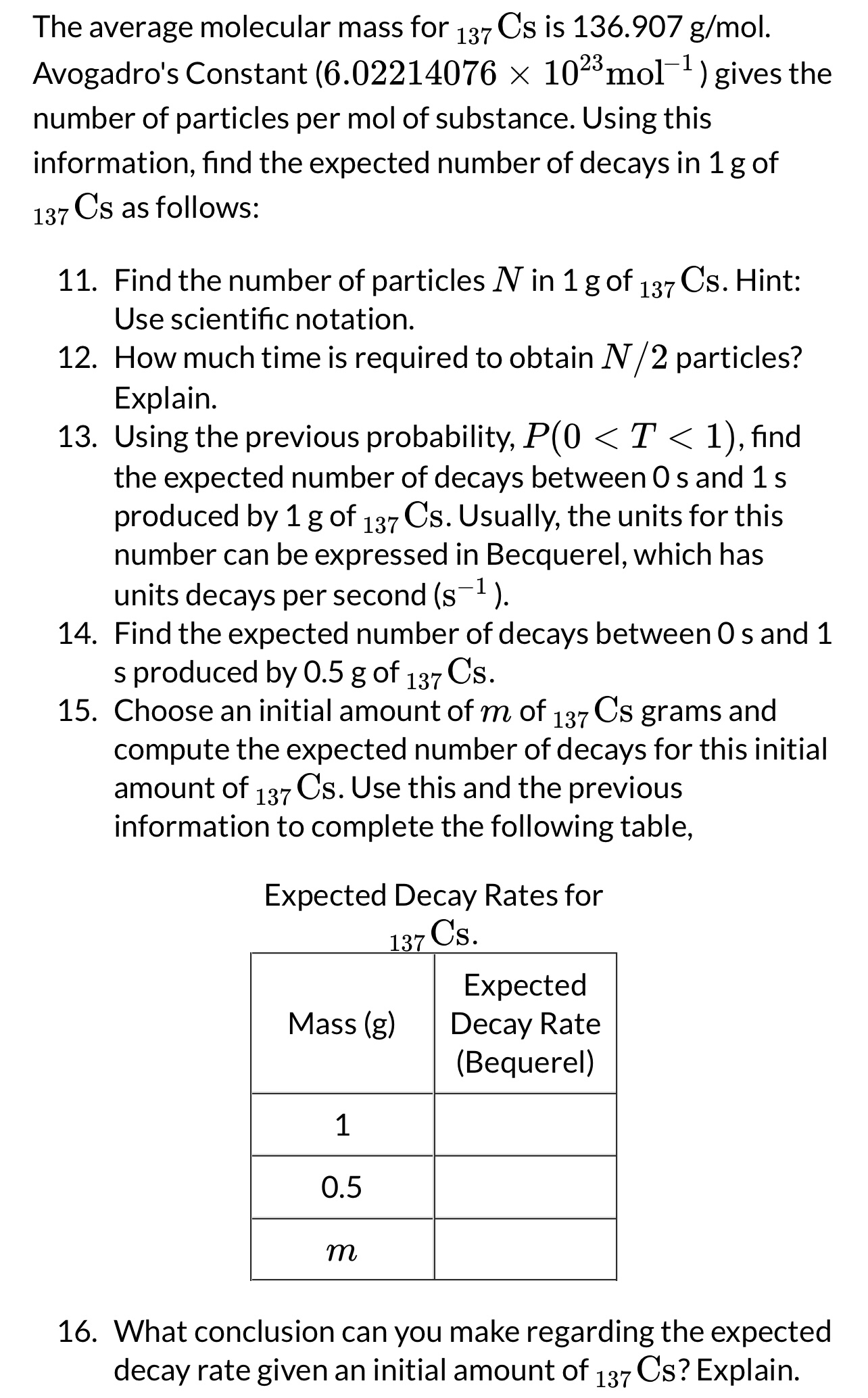
The average molecular mass for is Avogadro's Constant gives the number of particles per mol of substance. Using this information, find the expected number of decays in of as follows:
Find the number of particles in of Hint: Use scientific notation.
How much time is required to obtain particles? Explain.
Using the previous probability,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


