Question: The bicarbonate buffer system involves the balance of carbon dioxide, carbonic acid, and bicarbonate to maintain pH in the blood. CO2+H2OH2CO3HCO3++H+ On average, your blood
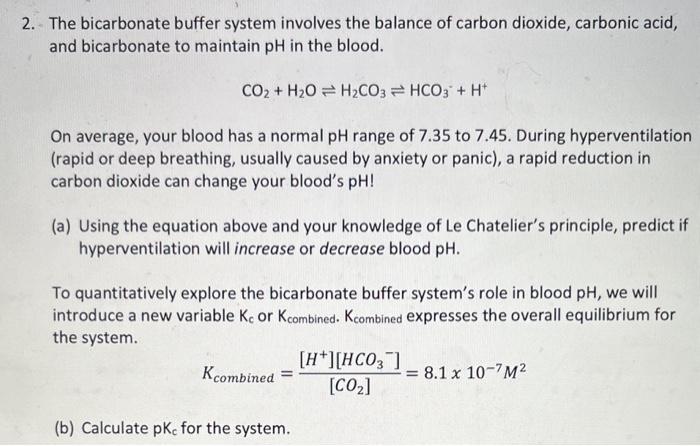
The bicarbonate buffer system involves the balance of carbon dioxide, carbonic acid, and bicarbonate to maintain pH in the blood. CO2+H2OH2CO3HCO3++H+ On average, your blood has a normal pH range of 7.35 to 7.45. During hyperventilation (rapid or deep breathing, usually caused by anxiety or panic), a rapid reduction in carbon dioxide can change your blood's pH ! (a) Using the equation above and your knowledge of Le Chatelier's principle, predict if hyperventilation will increase or decrease blood pH. To quantitatively explore the bicarbonate buffer system's role in blood pH, we will introduce a new variable Kc or Kcombined. Kcombined expresses the overall equilibrium for the system. Kcombined=[CO2][H+][HCO3]=8.1107M2 (b) Calculate pKc for the system
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


