Question: The binary system n-hexane (1)+ethanol (2) obeys to the modified Raoult's law with the following activity coefficients expressions: ln1=1.5x22;ln2=1.5x12 a) Show whether or not this
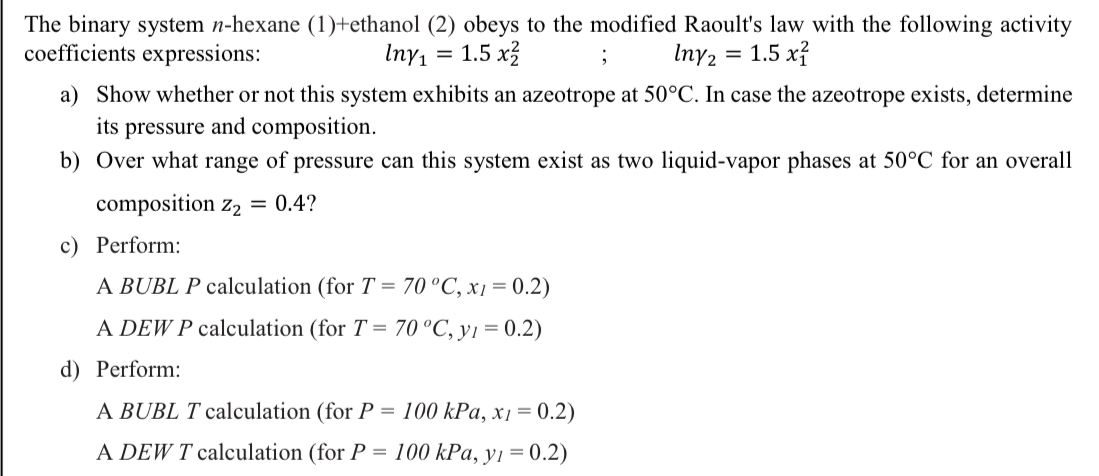
The binary system n-hexane (1)+ethanol (2) obeys to the modified Raoult's law with the following activity coefficients expressions: ln1=1.5x22;ln2=1.5x12 a) Show whether or not this system exhibits an azeotrope at 50C. In case the azeotrope exists, determine its pressure and composition. b) Over what range of pressure can this system exist as two liquid-vapor phases at 50C for an overall composition z2=0.4 ? c) Perform: A BUBLP calculation (for T=70C,xl=0.2 ) A DEWP calculation (for T=70C,yl=0.2 ) d) Perform: A BUBL T calculation (for P=100kPa,xI=0.2 ) A DEWT calculation (for P=100kPa,yl=0.2 )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


