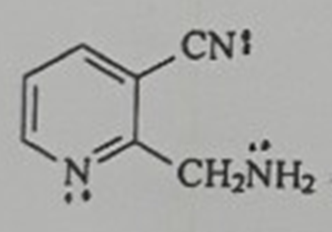
Question: The compound shown below contains three non - bonding pairs of electrons. Which one of these three pairs of electrons is the most stable (
The compound shown below contains three nonbonding pairs of electrons. Which one of these three pairs of electrons is the most stable lowest energy Which one is the least stable highest energy Why?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


