In worked example 2.7, the structure of the [XeF 5 ] ion was predicted. Confirm that
Question:
In worked example 2.7, the structure of the [XeF5]− ion was predicted. Confirm that this structure is consistent with D5h symmetry.
Data from Example 2.7.
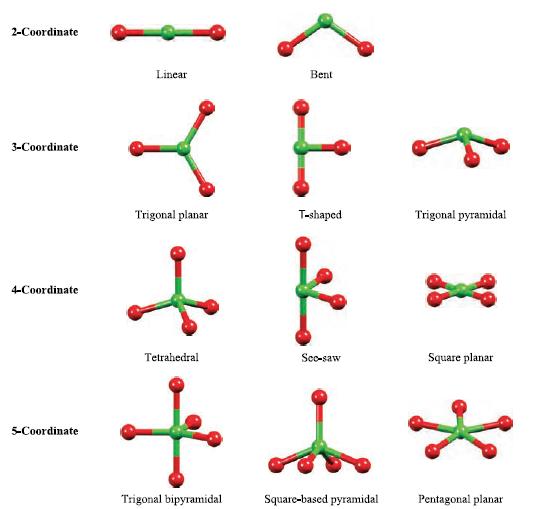
Predict the structures of (a) XeF2 and (b) [XeF5]− Xe is in group 18 and possesses eight electrons in its valence shell. F is in group 17, has seven valence electrons and forms one covalent single bond. Before applying the VSEPR model, decide which is the central atom in the molecule. In each of (a) XeF2 and (b) [XeF5]−, Xe is the central atom. (a) XeF2. Two of the eight valence electrons of the Xe atom are used for bonding (two Xe—F single bonds), and so around the Xe centre there are two bonding pairs of electrons and three lone pairs. The parent shape is a trigonal bipyramid (Fig. 2.16) with the three lone pairs in the equatorial plane to minimize lone pair– lone pair repulsions. The XeF2 molecule is therefore linear:
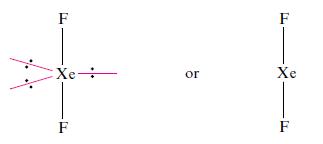
(b) [XeF5]−. The electron from the negative charge is conveniently included within the valence shell of the central atom. Five of the nine valence electrons are used for bonding and around the Xe centre there are five bonding pairs and two lone pairs of electrons. The parent shape is a pentagonal bipyramid (Fig. 2.16) with the two lone pairs opposite to each other to minimize lone pair–lone pair repulsions. The [XeF5]− anion is therefore pentagonal planar:
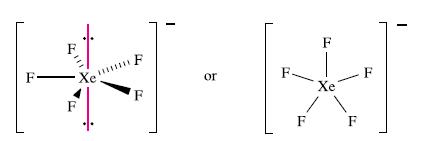
When structures are determined by diffraction methods, atom positions are effectively located. Thus, in terms of a molecular structure, XeF2 is linear and [XeF5]− is pentagonal planar. In the diagrams above, two representations of each species are shown, one with the lone pairs to emphasize the origin of the prediction from the VSEPR model.
Figure 2.16
Step by Step Answer:






