Question: The cumene process is a common method for the production of phenol and acetone. The overall reaction is as follows: C6H6+C3H6+O2C3H6O+C6H5OH For a feed of
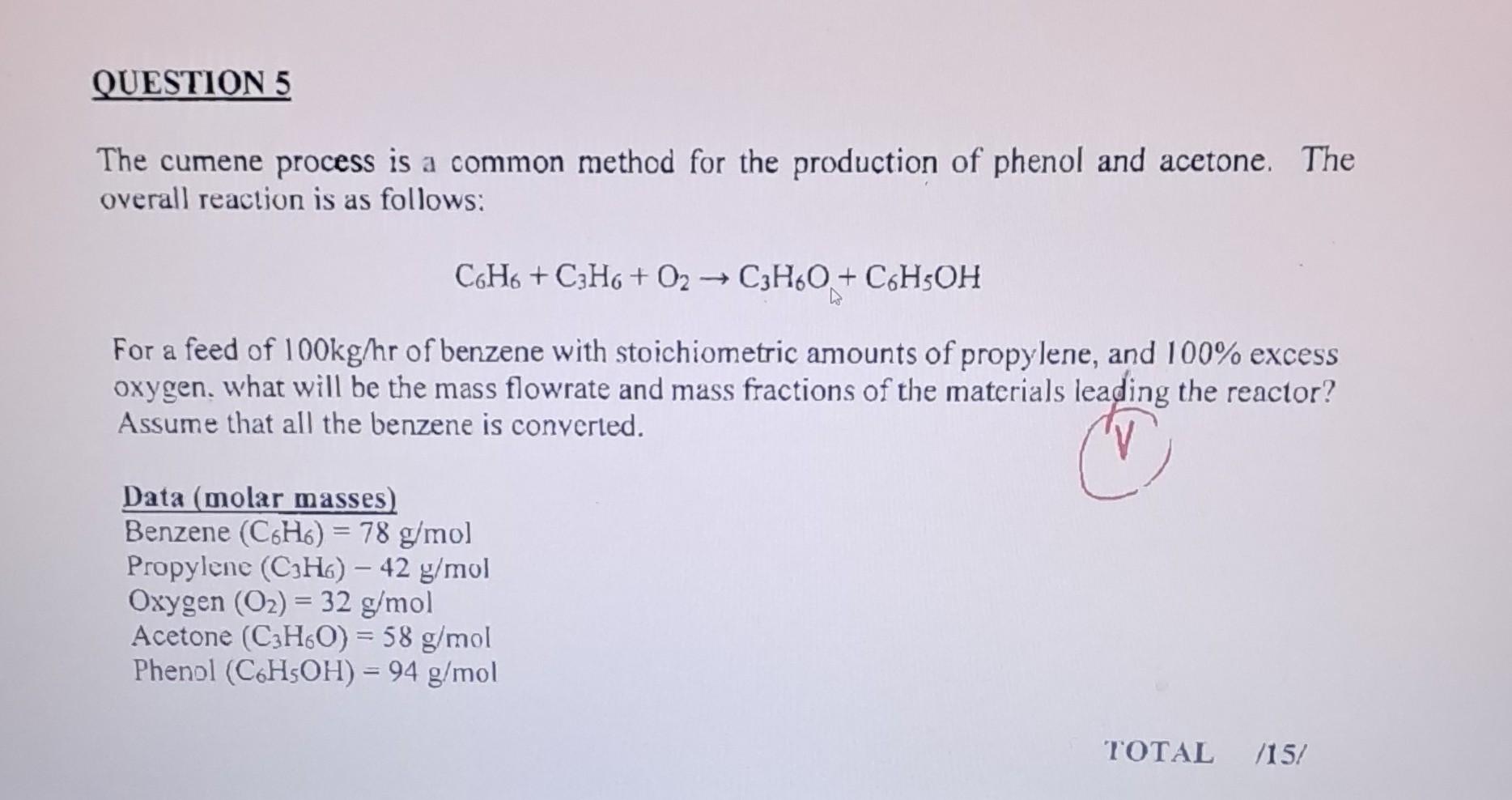
The cumene process is a common method for the production of phenol and acetone. The overall reaction is as follows: C6H6+C3H6+O2C3H6O+C6H5OH For a feed of 100kg/hr of benzene with stoichiometric amounts of propylene, and 100% excess oxygen. what will be the mass flowrate and mass fractions of the materials leading the reactor? Assume that all the benzene is converted. Data (molar masses) Benzene (C6H6)=78g/mol Propylene (C3H6)42g/mol Oxygen (O2)=32g/mol Acetone (C3H6O)=58g/mol Phenol (C6H5OH)=94g/mol The cumene process is a common method for the production of phenol and acetone. The overall reaction is as follows: C6H6+C3H6+O2C3H6O+C6H5OH For a feed of 100kg/hr of benzene with stoichiometric amounts of propylene, and 100% excess oxygen. what will be the mass flowrate and mass fractions of the materials leading the reactor? Assume that all the benzene is converted. Data (molar masses) Benzene (C6H6)=78g/mol Propylene (C3H6)42g/mol Oxygen (O2)=32g/mol Acetone (C3H6O)=58g/mol Phenol (C6H5OH)=94g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


