Question: The data below is for the reaction sequence A-B-C-D Elementary step A,H/kJ mol E/kl mol A B 22 33 B1C -11 22 21D -33
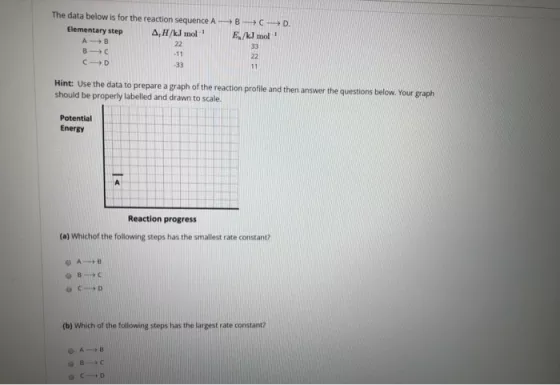
The data below is for the reaction sequence A-B-C-D Elementary step A,H/kJ mol E/kl mol A B 22 33 B1C -11 22 21D -33 11 Hint: Use the data to prepare a graph of the reaction profile and then answer the questions below. Your graph should be properly labelled and drawn to scale. Potential Energy Reaction progress (a) Which of the following steps has the smallest rate constant? 6810 2119 (b) Which of the following steps has the largest rate constant? DA 9216 |
Step by Step Solution
3.40 Rating (150 Votes )
There are 3 Steps involved in it
a Since k AeEaRT Higner the Ea lesser is the ... View full answer

Get step-by-step solutions from verified subject matter experts


