Question: The initial reaction rate for the elementary reaction A+2B 3C was measured as a function of temperature when the concentration of A was 2.5
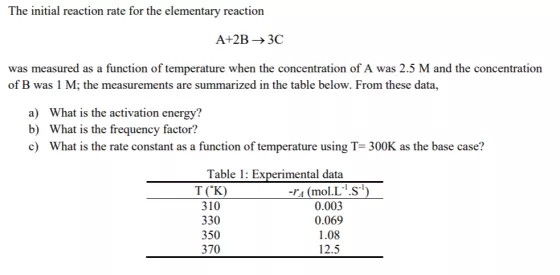
The initial reaction rate for the elementary reaction A+2B 3C was measured as a function of temperature when the concentration of A was 2.5 M and the concentration of B was 1 M; the measurements are summarized in the table below. From these data, a) What is the activation energy? b) What is the frequency factor? c) What is the rate constant as a function of temperature using T= 300K as the base case? Table 1: Experimental data T(K) -Ya (mol.L".S"') 0.003 310 330 350 0.069 1.08 370 12.5
Step by Step Solution
3.43 Rating (172 Votes )
There are 3 Steps involved in it
Solution Frem Arrhenius equation 1 K Ko e ERT where k is geat... View full answer

Get step-by-step solutions from verified subject matter experts


