Question: The data below refer to the anodic current through a platinum electrode of area 2.0cm2 in -contact with an Fe3+,Fe2+ aqueous solution at 298K. Calculate
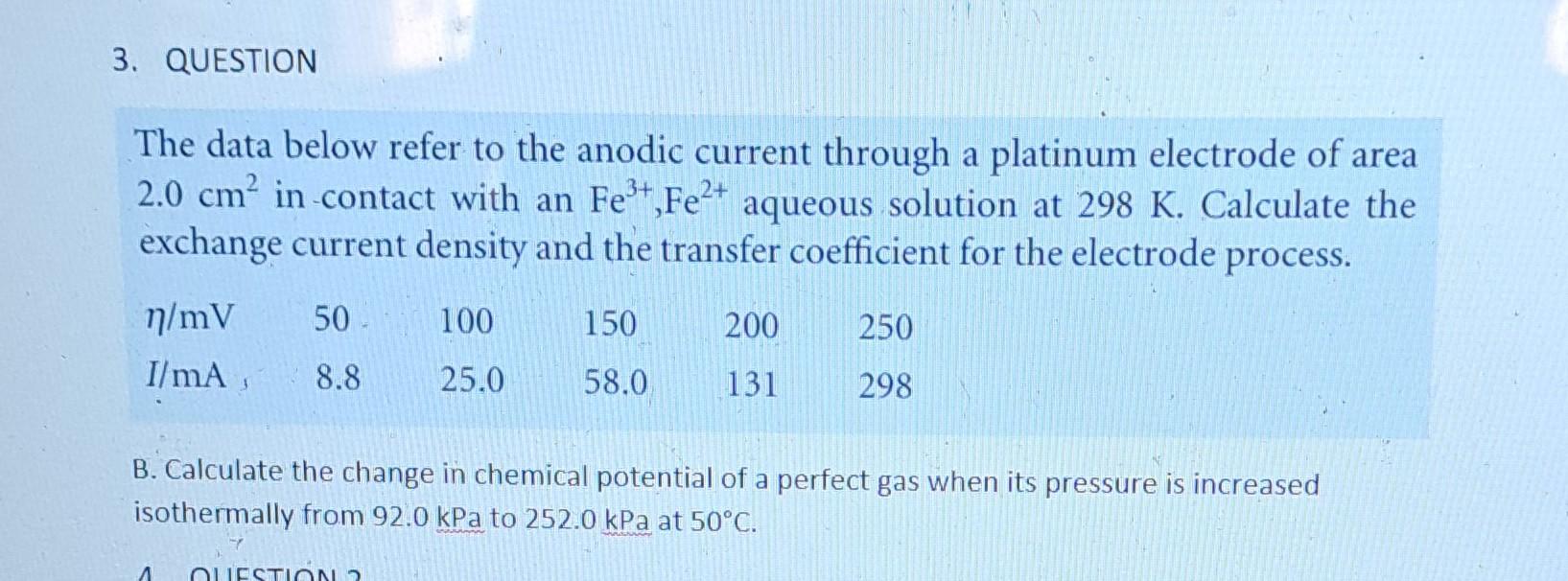
The data below refer to the anodic current through a platinum electrode of area 2.0cm2 in -contact with an Fe3+,Fe2+ aqueous solution at 298K. Calculate the exchange current density and the transfer coefficient for the electrode process. B. Calculate the change in chemical potential of a perfect gas when its pressure is increased isothermally from 92.0kPa to 252.0kPa at 50C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


