Question: Materials Science & Engineering Problem! (Chemical Engineering) Please answer this question as soon as possible with specific explanation. If you're going to write it in
Materials Science & Engineering Problem! (Chemical Engineering)
Please answer this question as soon as possible with specific explanation.
If you're going to write it in handwriting, please write it clearly so I can recognize it. I don't want you to write solution in cursive letter.
I can give you thumbs up when your answer is correct.
Thanks.

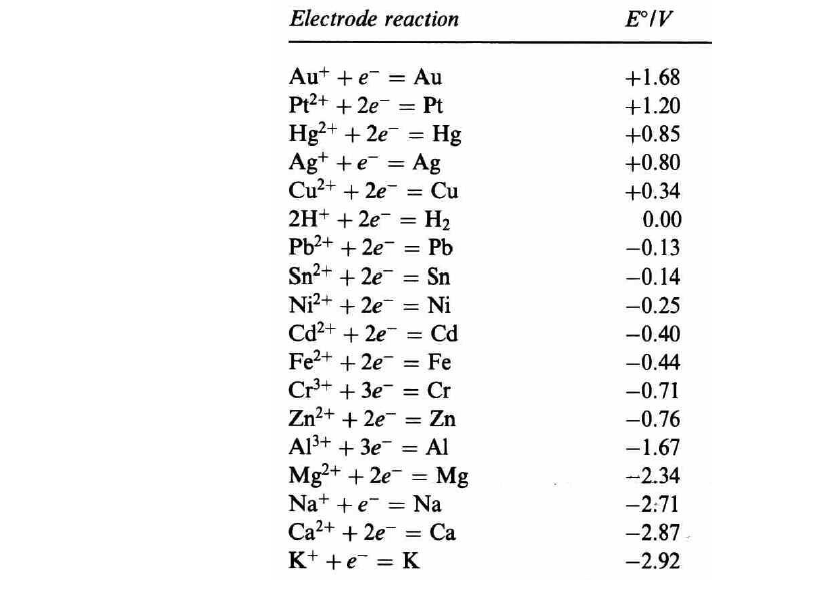
Question 2 (1.5% Final Score): Congratulations! After solving Question 1 your prospects for a promotion in the factory are looking great! Unfortunately, there are many problems in the old factory and this time you must analytically calculate the corrosion potential (Ecorr) and the corrosion current density (corr) (1% Final Score), and the protection potential (Eprotection) and the protection current density (Iprotection) (0.5% Final Score) of an electrode of zinc (Zn) immersed in an aqueous solution of hydrochloric acid (HCI) 1 M under standard conditions. To help you, your fantastic team have (again) performed a series of preliminary experiments to collect some critical data about this system: . Finding 1: The cathodic (Pc) and anodic (Ba) Tafel slopes of the corrosion process in the electrode are c =-0.15 V/decade of current density and Ba = 0.15 V/decade of current density. Finding 2: The exchange current densities for the cathodic and anodic reactions are iH2 = 2.10-4 A cm- and izn = 2.10-5 A cm-2 NB: [H+] = 1.10 PH and Ecorr potential at equilibrium vs SHE. - . = Electrode reaction E/V + = Aut += Au Pt2+ + 2e = Pt Hg2+ + 2e = Hg Ag+ + = Ag te Cu2+ + 2e = Cu 2H+ + 2e = H2 Pb2+ + 2e = Pb - Sn2+ + 2e = Sn Ni2+ + 2e = Ni Cd2+ + 2e = Cd Fe2+ + 2e = Fe Cr3+ + 3e = Cr Zn2+ + 2e = Zn + A13+ + 3e = Al Mg2+ + 2e = Mg Na+ += Na e Ca2+ + 2e = Ca K+ + = K +1.68 +1.20 +0.85 +0.80 +0.34 0.00 -0.13 -0.14 -0.25 -0.40 -0.44 -0.71 -0.76 -1.67 -2.34 -2.71 -2.87 -2.92 = = =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


