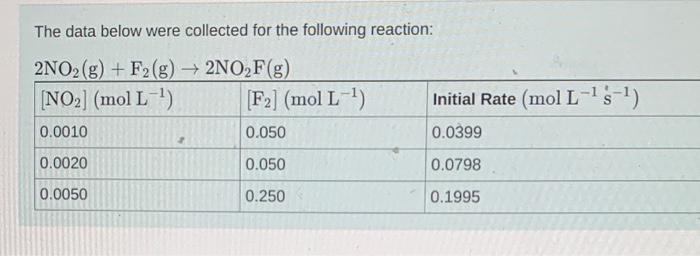
Question: The data below were collected for the following reaction: Write an expression for the reaction rate law. Rate=k[NO2]Rate=k[F2]Rate=k[NO2][F2]Rate=k[NO2]2[F2] You have already submitted this answer. Enter
![expression for the reaction rate law. Rate=k[NO2]Rate=k[F2]Rate=k[NO2][F2]Rate=k[NO2]2[F2] You have already submitted this](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8ff771c045_35866f8ff76a172c.jpg)
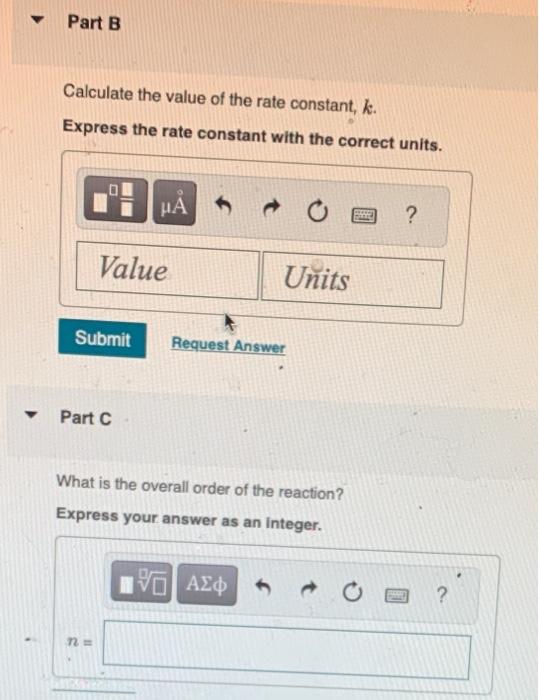
The data below were collected for the following reaction: Write an expression for the reaction rate law. Rate=k[NO2]Rate=k[F2]Rate=k[NO2][F2]Rate=k[NO2]2[F2] You have already submitted this answer. Enter a new answer. No credit lost. Try again. Calculate the value of the rate constant, k. Express the rate constant with the correct units. Part C What is the overall order of the reaction? Express your answer as an integer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


