Question: The data provided below (Table 1) were collected for the following reaction at 904C: 2NO(g) + 2H2(g) - N2(g) + 2H2O(g) Table l: Experimental data
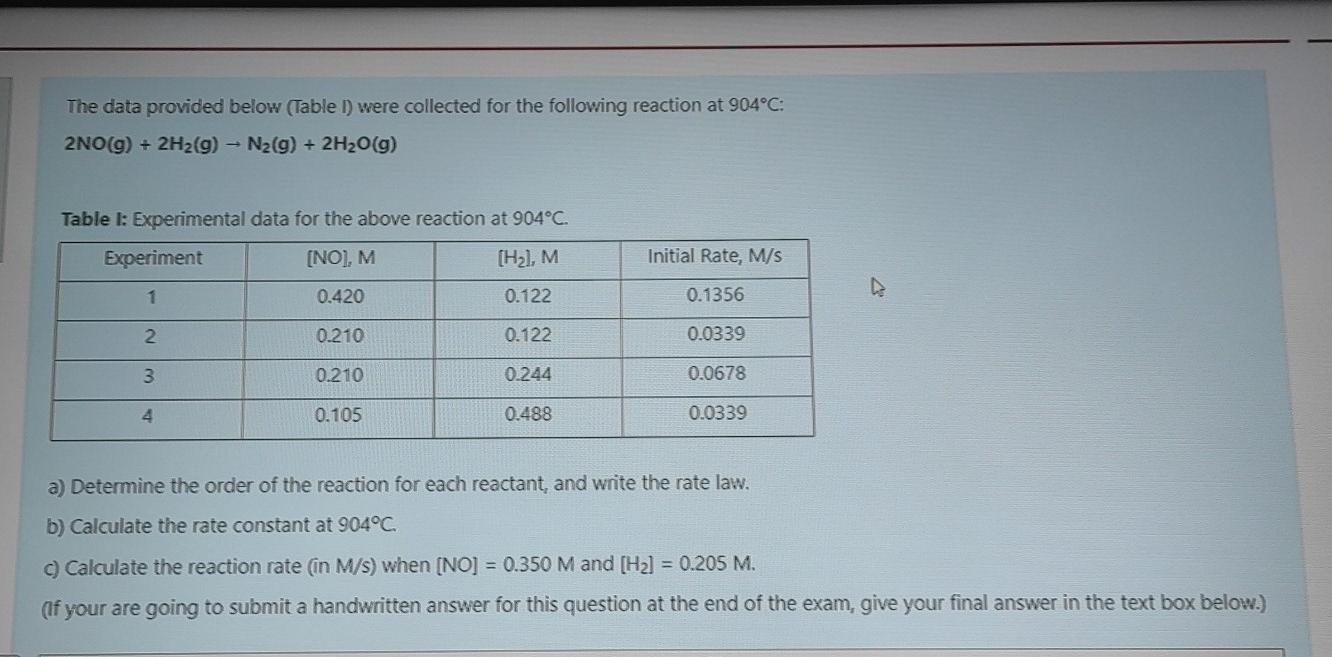
The data provided below (Table 1) were collected for the following reaction at 904C: 2NO(g) + 2H2(g) - N2(g) + 2H2O(g) Table l: Experimental data for the above reaction at 904C. Experiment [NO], M (Hal, M Initial Rate, M/S 1 0.420 0.122 0.1356 2 0.210 0.122 0.0339 3 0.210 0.244 0.0678 0.105 0.488 0.0339 a) Determine the order of the reaction for each reactant, and write the rate law. b) Calculate the rate constant at 904C. c) Calculate the reaction rate in M/s) when [NO] = 0.350 M and (H2] = 0.205 M. (If your are going to submit a handwritten answer for this question at the end of the exam, give your final answer in the text box below.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


