Question: The data in the following table are adsorption amounts according to pressure obtained by adsorbing nitrogen at 77 K to measure the surface area of
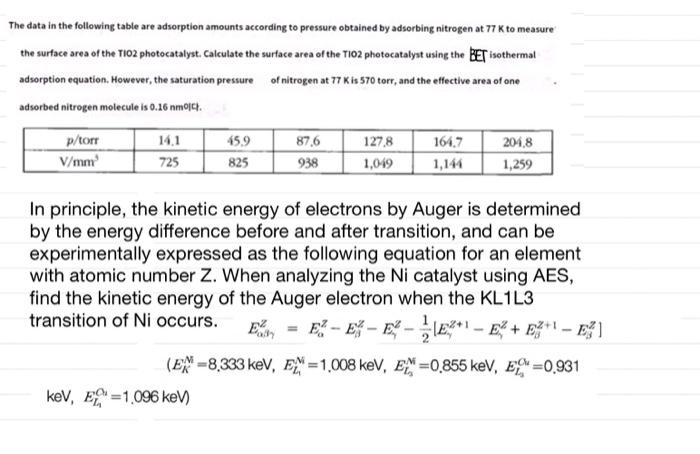
The data in the following table are adsorption amounts according to pressure obtained by adsorbing nitrogen at 77 K to measure the surface area of the TiO2 photocatalyst Calculate the surface area of the TiO2 photocatalyst using the BET isothermal adsorption equation. However, the saturation pressure of nitrogen at 77 Kis 570 torr, and the effective area of one adsorbed nitrogen molecule is 0.26 nmoch p/torr 141 87.6 1278 164.7 204,8 V/mm 725 825 938 1,019 1,144 1,259 45.9 In principle, the kinetic energy of electrons by Auger is determined by the energy difference before and after transition, and can be experimentally expressed as the following equation for an element with atomic number Z. When analyzing the Ni catalyst using AES, find the kinetic energy of the Auger electron when the KL1L3 transition of Ni occurs. E = E? - E* - ES - EX*! E% + EZ"! ] (EX-8.333 keV, EX=1,008 keV, E =0.855 keV, EX=0,931 keV, E =1,096 keV)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


