Question: The dilution factor is the number by which you must multiply the original concentration to find the concentration of the diluted solution. The dilution

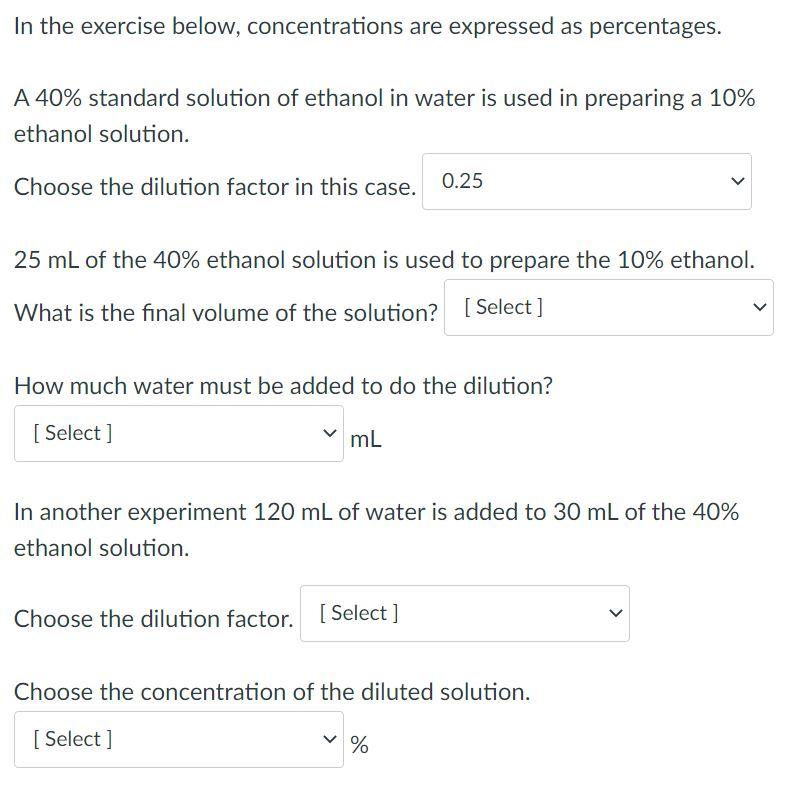
The dilution factor is the number by which you must multiply the original concentration to find the concentration of the diluted solution. The dilution factor is calculated as below. dilution factor = concentration of diluted concentration of original The dilution factor is also equal to the ratio of the volumes of the original and diluted solutions. Because the diluted solution has a larger volume, it is now in the denominator. dilution factor = volume of original volume of diluted In the exercise below, concentrations are expressed as percentages. A 40% standard solution of ethanol in water is used in preparing a 10% ethanol solution. Choose the dilution factor in this case. 0.25 25 mL of the 40% ethanol solution is used to prepare the 10% ethanol. What is the final volume of the solution? [Select] How much water must be added to do the dilution? mL [Select] In another experiment 120 mL of water is added to 30 mL of the 40% ethanol solution. Choose the dilution factor. [Select] Choose the concentration of the diluted solution. [ Select] %
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


