Question: The dimer component undergoes an elementary decomposition reaction to a gaseous monomer component (M) according to the reaction: D 2 M. The critical temperature
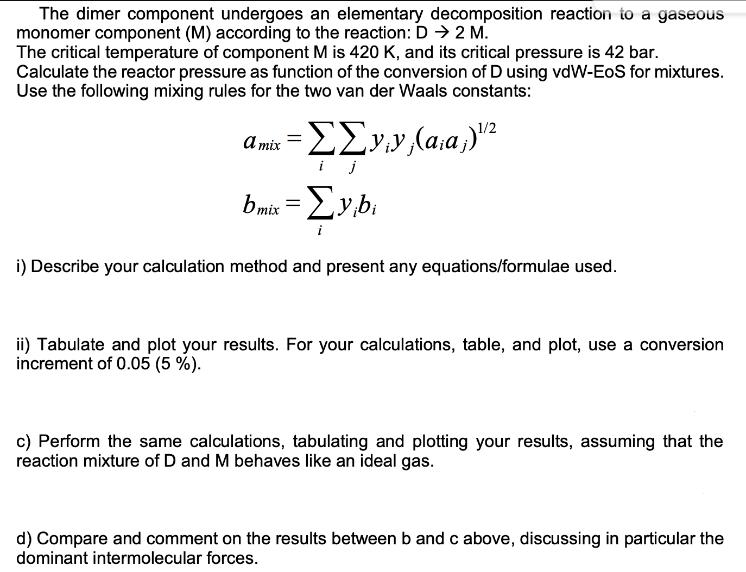
The dimer component undergoes an elementary decomposition reaction to a gaseous monomer component (M) according to the reaction: D 2 M. The critical temperature of component M is 420 K, and its critical pressure is 42 bar. Calculate the reactor pressure as function of the conversion of D using vdW-EOS for mixtures. Use the following mixing rules for the two van der Waals constants: a mix = (:;)12 i j bmix = ybi i) Describe your calculation method and present any equations/formulae used. ii) Tabulate and plot your results. For your calculations, table, and plot, use a conversion increment of 0.05 (5 %). c) Perform the same calculations, tabulating and plotting your results, assuming that the reaction mixture of D and M behaves like an ideal gas. d) Compare and comment on the results between b and c above, discussing in particular the dominant intermolecular forces. The dimer component undergoes an elementary decomposition reaction to a gaseous monomer component (M) according to the reaction: D 2 M. The critical temperature of component M is 420 K, and its critical pressure is 42 bar. Calculate the reactor pressure as function of the conversion of D using vdW-EOS for mixtures. Use the following mixing rules for the two van der Waals constants: a mix = (:;)12 i j bmix = ybi i) Describe your calculation method and present any equations/formulae used. ii) Tabulate and plot your results. For your calculations, table, and plot, use a conversion increment of 0.05 (5 %). c) Perform the same calculations, tabulating and plotting your results, assuming that the reaction mixture of D and M behaves like an ideal gas. d) Compare and comment on the results between b and c above, discussing in particular the dominant intermolecular forces.
Step by Step Solution
There are 3 Steps involved in it
i Calculation Method and Equations To calculate the reactor pressure as a function of the conversion of D we will use the van der Waals equation of st... View full answer

Get step-by-step solutions from verified subject matter experts


