Question: The element that composes seawater along with the corresponding presents are shown in the table if a sample of seawater contains 35 mL how many
The element that composes seawater along with the corresponding presents are shown in the table if a sample of seawater contains 35 mL how many milliliters of oxygen are in the sample?
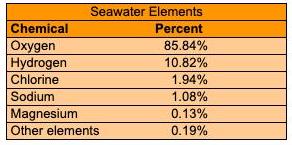
There are __ ml of oxygen in the sample. (Type an integer or a decimal to two decimal places).
Seawater Elements Chemical Percent Oxygen Hydrogen 85.84% 10.82% Chlorine 1.94% Sodium 1.08% Magnesium 0.13% Other elements 0.19%
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Given data Sample of sea water 35 ml Sea water sam... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


