Question: The elemental analysis for an unknown compound shows: C: 36.7%; H: 6.6%; O: 16.3% . Determine the structure of this compound and explain the bond
The elemental analysis for an unknown compound shows: C: 36.7%; H: 6.6%; O: 16.3% . Determine the structure of this compound and explain the bond cleavage mechanism that produces the fragment ion peaks at m/z = 151/153.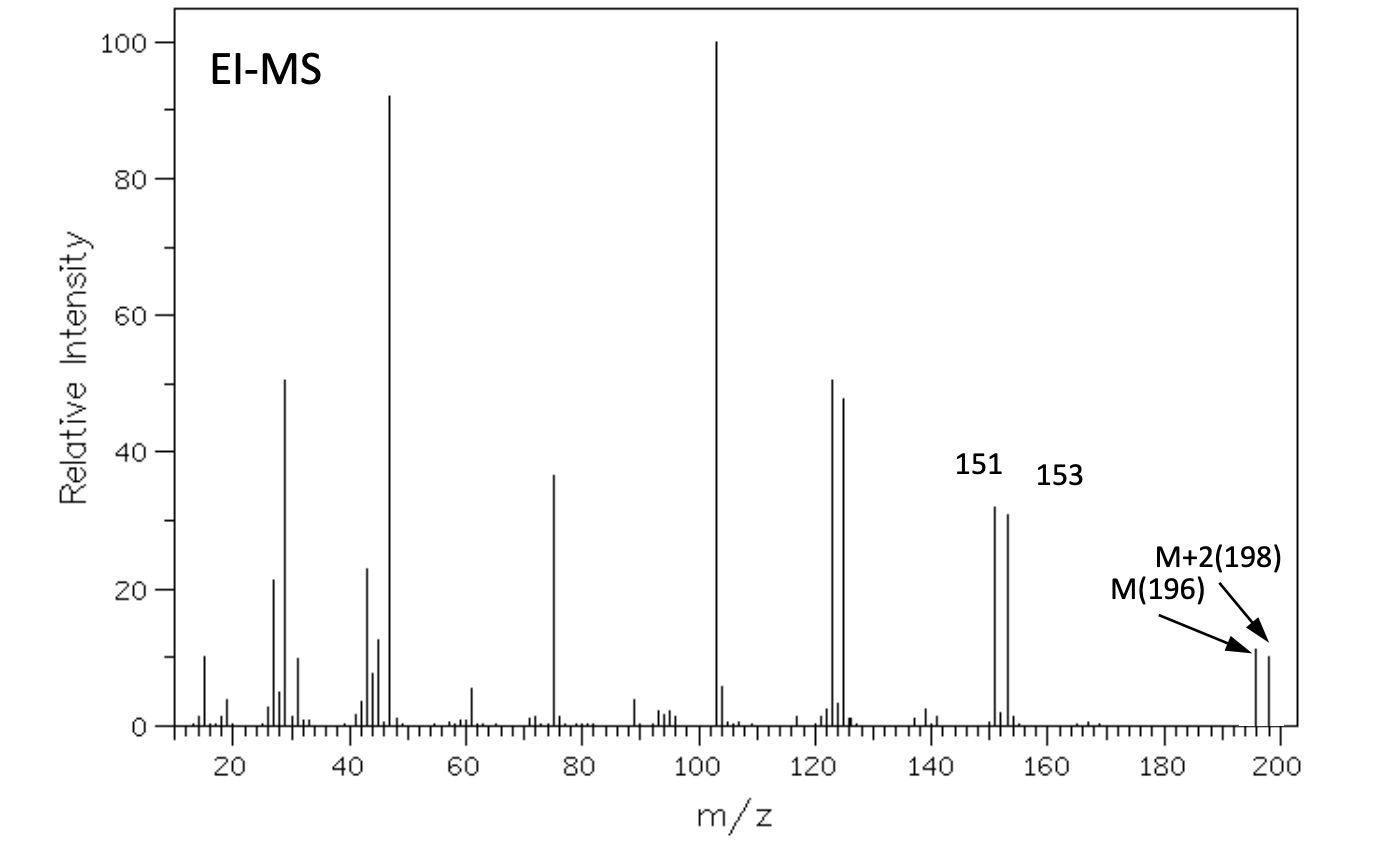
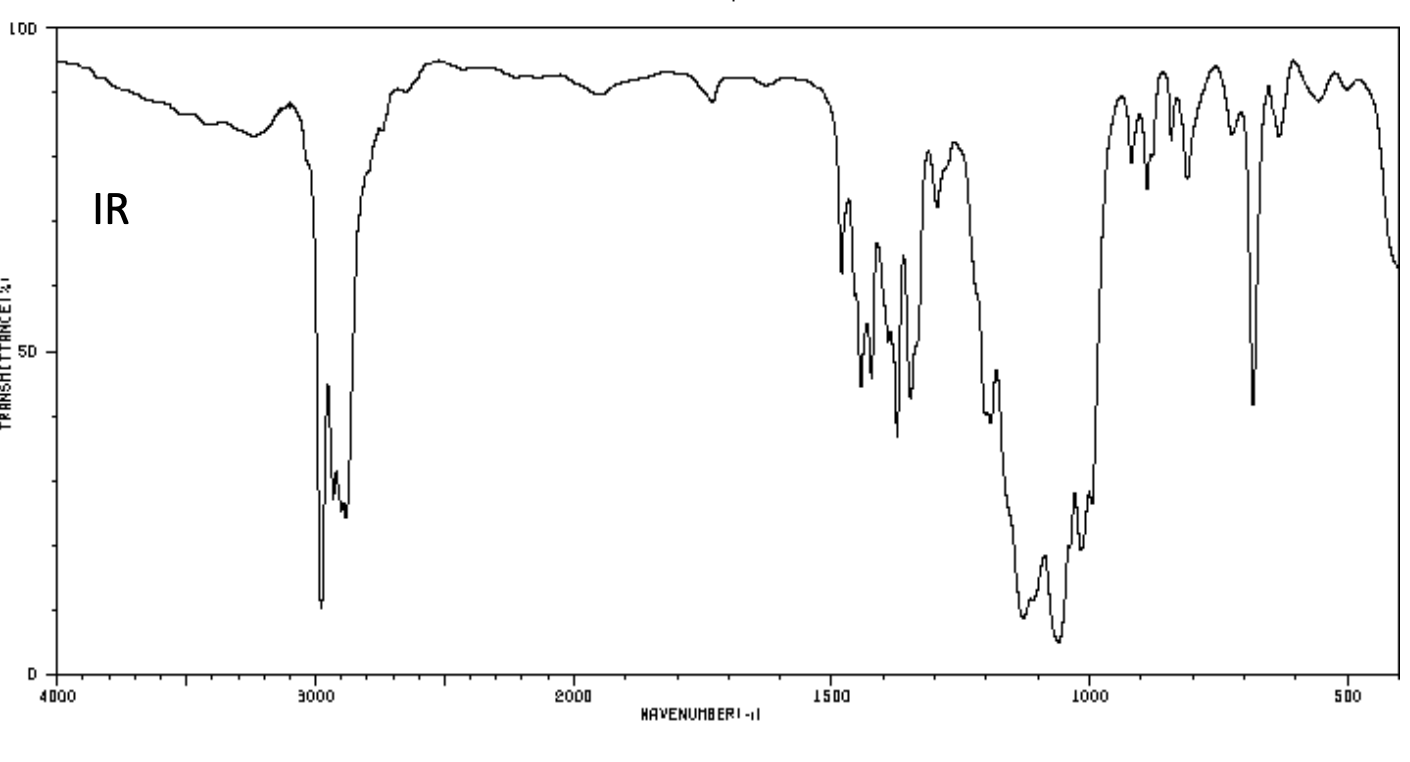
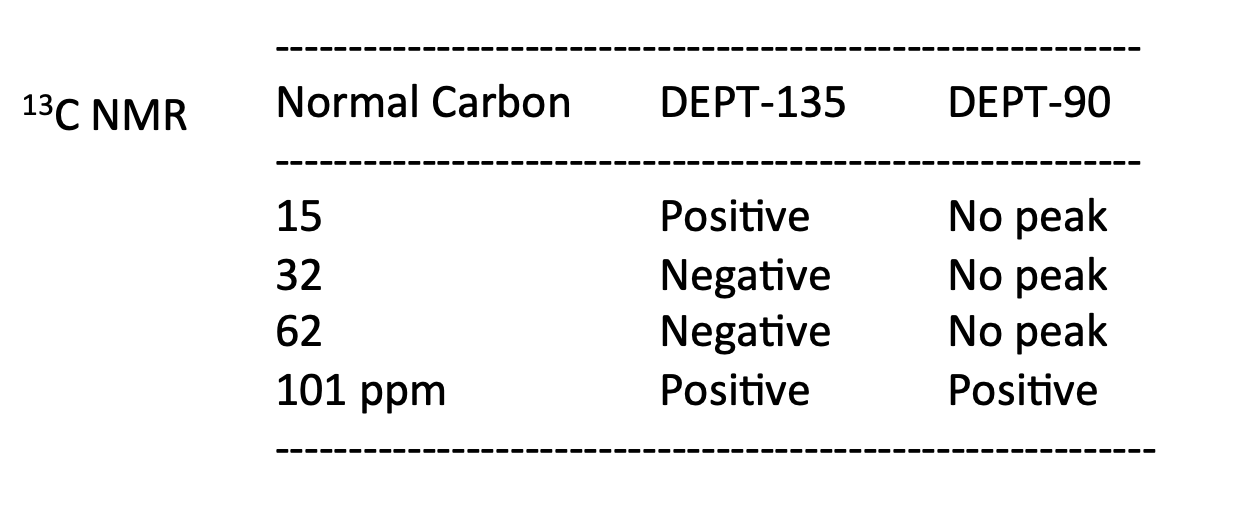
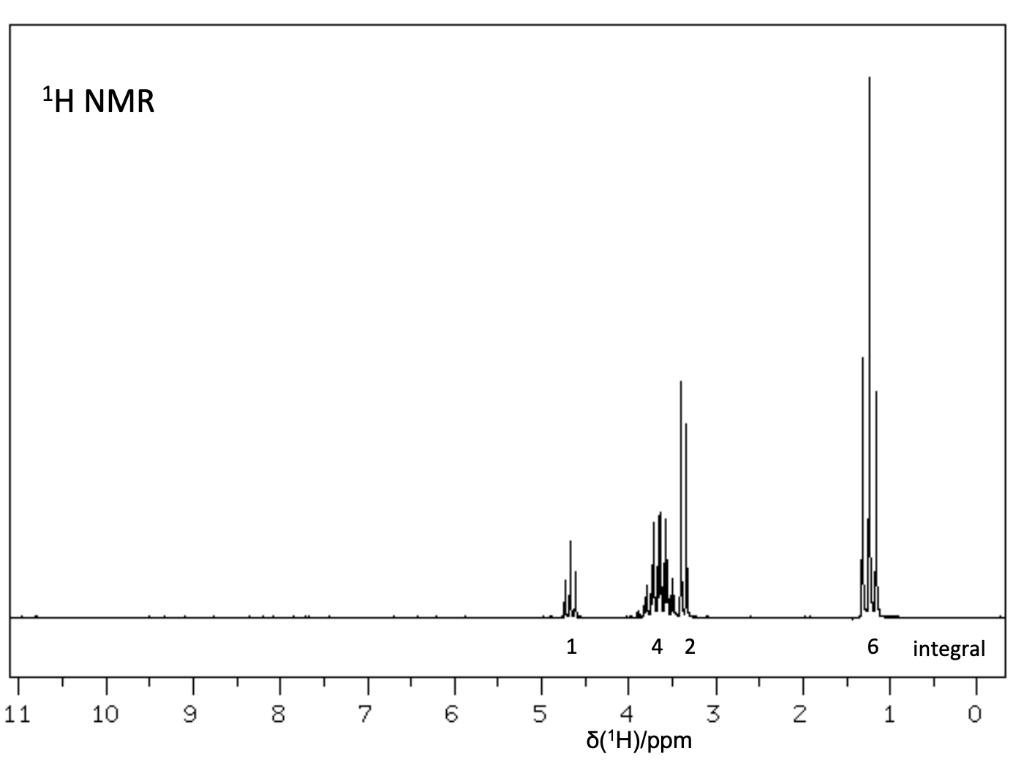
100 El-MS 80 T 60 Relative Intensity 40 151 153 M+2(198) M(196) 20 th 100 20 40 60 80 120 140 160 180 200 m/z LOD W IR D 4000 3000 2000 1500 1000 500 HAVENUHBERI - 13C NMR Normal Carbon DEPT-135 DEPT-90 15 32 62 Positive Negative Negative Positive No peak No peak No peak Positive 101 ppm 1H NMR 1 4 2 6 integral T 1 ! T T T 11 10 9 8 7 0) 5 3 2 1 0 4 8('H)/ppm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


