Question: The elementary isomerization reaction AB is taking place inside a porous catalyst. The reaction is first order, rA=kcA. If the particle has a slab ()
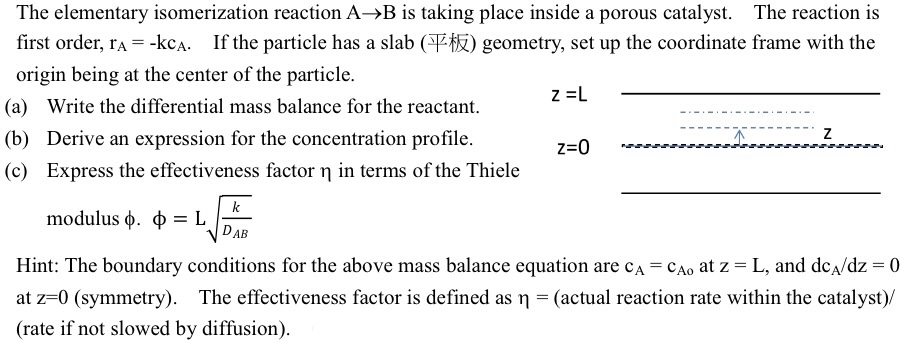
The elementary isomerization reaction AB is taking place inside a porous catalyst. The reaction is first order, rA=kcA. If the particle has a slab () geometry, set up the coordinate frame with the origin being at the center of the particle. (a) Write the differential mass balance for the reactant. z=L (b) Derive an expression for the concentration profile. z=0 (c) Express the effectiveness factor in terms of the Thiele modulus .=LDABk Hint: The boundary conditions for the above mass balance equation are cA=cAo at z=L, and dcA/dz=0 at z=0 (symmetry). The effectiveness factor is defined as = (actual reaction rate within the catalyst)/ (rate if not slowed by diffusion)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


