Question: The elementary liquid-phase reaction: A + B C is to be carried out in a CSTR with three impellers. The mixing pattern in the
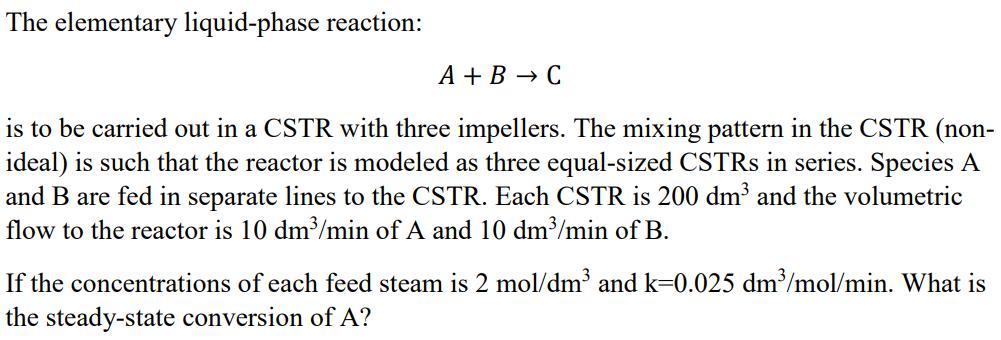
The elementary liquid-phase reaction: A + B C is to be carried out in a CSTR with three impellers. The mixing pattern in the CSTR (non- ideal) is such that the reactor is modeled as three equal-sized CSTRs in series. Species A and B are fed in separate lines to the CSTR. Each CSTR is 200 dm and the volumetric flow to the reactor is 10 dm/min of A and 10 dm/min of B. If the concentrations of each feed steam is 2 mol/dm and k=0.025 dm/mol/min. What is the steady-state conversion of A?
Step by Step Solution
There are 3 Steps involved in it
To find the steadystate conversion of A in the CSTR system we need to analyze the reaction kinetics and the flow rates in each CSTR Given The CSTR sys... View full answer

Get step-by-step solutions from verified subject matter experts


