Question: The elementary reaction 2H2O(g)2H2(g)+O2(g) proceeds at a certain temperature until the partial pressures of H2O,H2, and O2 reach 0.0700 atm, 0.00700 atm, and 0.00250 atm,
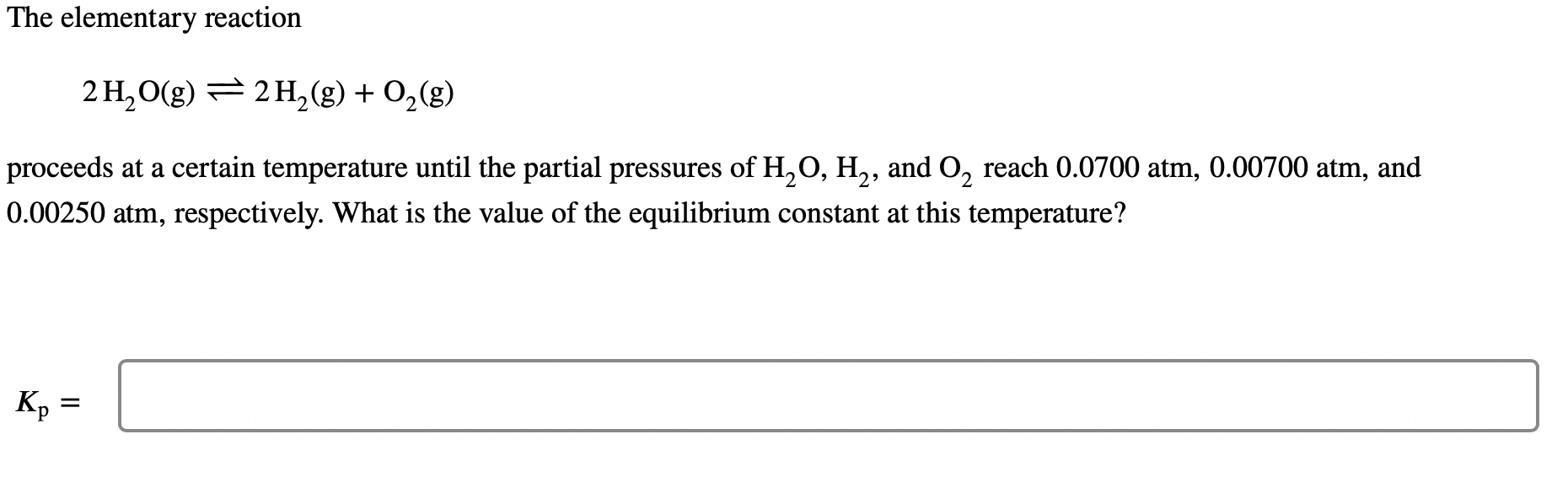
The elementary reaction 2H2O(g)2H2(g)+O2(g) proceeds at a certain temperature until the partial pressures of H2O,H2, and O2 reach 0.0700 atm, 0.00700 atm, and 0.00250 atm, respectively. What is the value of the equilibrium constant at this temperature? Kp=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


