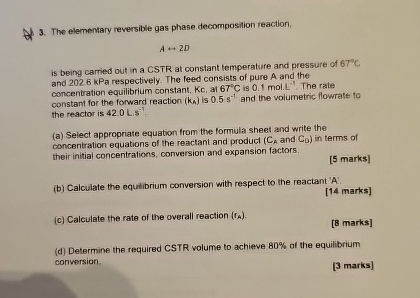
Question: The elementary reversible gas phase decomposition reaction. Aharr 2 D is being carried out in a CSTR at constant temperature and pressure of 6 7
The elementary reversible gas phase decomposition reaction.
Aharr
is being carried out in a CSTR at constant temperature and pressure of and kPa respectively. The leed consists of pure A and the concentration equilibrium constant, at is mol. The rate constant for the forward reaction is and the wolumetric flowrate to the reactor is
a Select appropriate equation from the formula sheet and write the concentration equations of the reactant and product and in terms of their initial concentrations, conversion and expansion factors.
marks
b Calculate the equilibrium conversion with respect to the reactant A:
marks
c Calculate the rate of the overall reaction
marks
d Determine the required CSTR volume to achieve of the equilibrium conversion.
marks
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


