Question: The equation below represents two processes that take place without any change in temperature I. HO(s) HO (1) II. CdCL 2(s) Cd+ (1) +
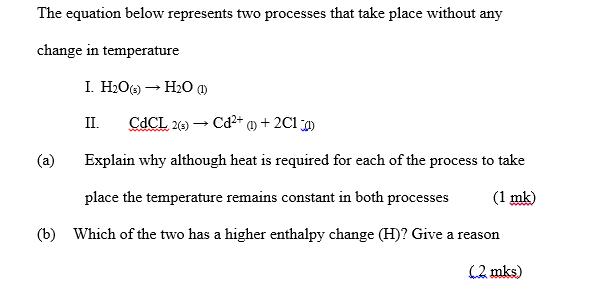
The equation below represents two processes that take place without any change in temperature I. HO(s) HO (1) II. CdCL 2(s) Cd+ (1) + 2C1) Explain why although heat is required for each of the process to take (1 mk) place the temperature remains constant in both processes (b) Which of the two has a higher enthalpy change (H)? Give a reason (2. mks) (a)
Step by Step Solution
3.42 Rating (165 Votes )
There are 3 Steps involved in it
a In both processes I HOs HOg and II CdCL2s Cd 1 2Cl1 the heat that is added is b... View full answer

Get step-by-step solutions from verified subject matter experts


