Question: The equation for photon energy, E, is E = he where h = 6.626x10-34 Js (Planck's constant) and C = 2.99x108 m/s (the speed of
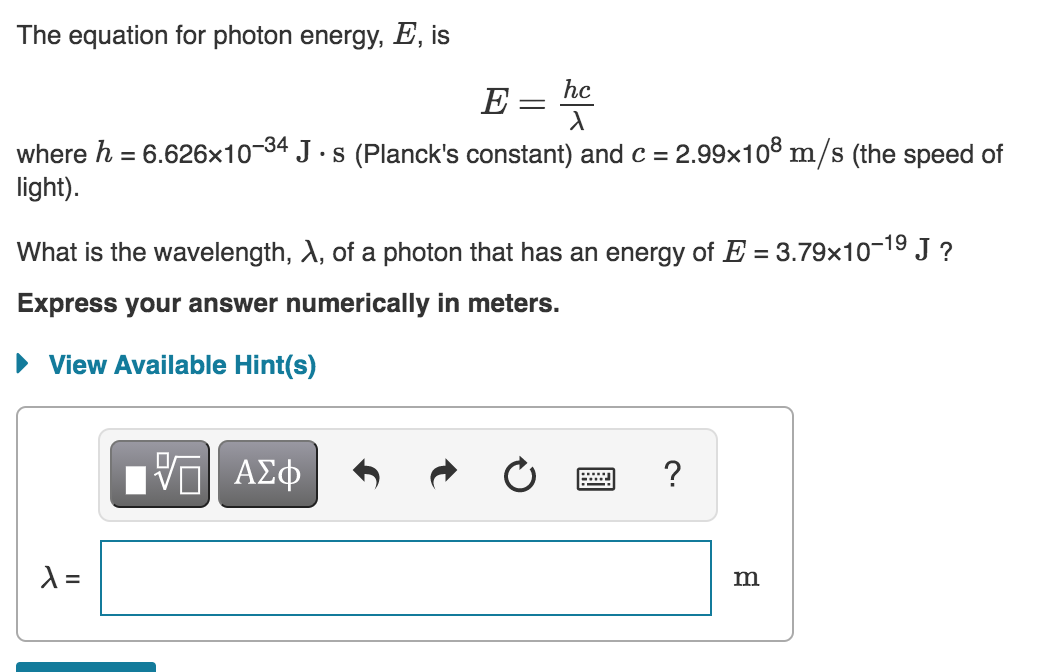
The equation for photon energy, E, is E = he where h = 6.626x10-34 Js (Planck's constant) and C = 2.99x108 m/s (the speed of light). What is the wavelength, 1, of a photon that has an energy of E = 3.79x10-19 J ? Express your answer numerically in meters. View Available Hint(s) VALO ? l= m
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


