Question: The exact same procedure outlined in the lab manual has been followed, Caiculate the mole ratio of OH/Ca2 of calcium hydroxide for the back titration
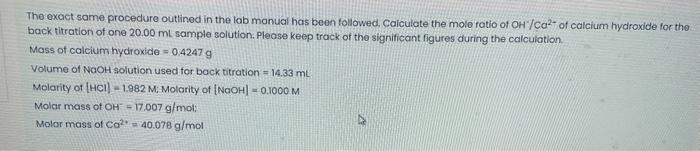

The exact same procedure outlined in the lab manual has been followed, Caiculate the mole ratio of OH/Ca2 of calcium hydroxide for the back titration of one 20.00mL sample solution. Please keep track of the significant figures during the calculation. Mass of calcium hydroxide =0.4247g Volume of NaOH solution used for back titration =14.33mL. Molarity of [HCl]=1.982M: Molarity of [NaOH]=0.1000M Molar mass of OH=17.007g/mol : Molar mass of Ca2+=40.078g/mol Procedure 1. Pre-weigh accurately 0.5g0.1g of calcium hydroxide into a 50mL beaker. Clean up any spills on the balance pan and around the balance. 2. Pipet 15.00mL of 2.0MHCl solution (record the exact molarity) into the 50mL beaker containing the calcium hydroxide. When all of the calcium hydroxide has dissolved, transfer the solution quantitatively to a 250.0mL volumetric flask and dilute it to volume with distilled water. 3. Into each of three Erlenmeyer flasks, pipet 20.00mL of your solution prepared in step \#2. 4. Add 2-3 drops of the phenolphthalein indicator to each of the three flasks. 5. Set up a buret as you have previously done. Clean and rinse the buret with a few millilitres of standard NaOH solution. Record the exact molarity of the standard NaOH solution. Fill the buret with the standard NaOH solution. 6. Titrate the excess HCl in each flask with the standard NaOH solution until you reach the endpoint. The endpoint is reached when the solution remains barely pink for at least 15 seconds. 7. Record the volume of NaOH used in the back titration for each of the three samples
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


