Question: The exchange current densities for a hydrogen-evolution reaction on three different metals are 5 x 10-7 A/cm, 3 x 10-7 A/cm, and 5 x 10-11
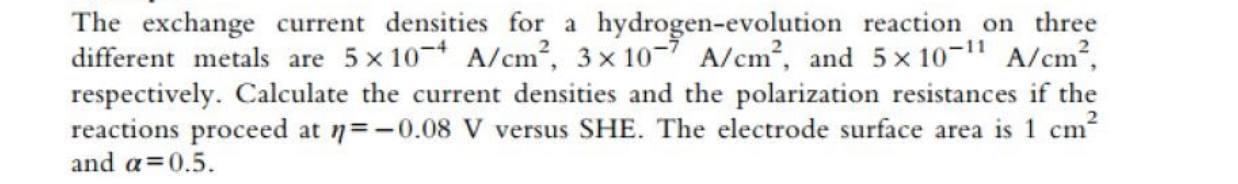
The exchange current densities for a hydrogen-evolution reaction on three different metals are 5 x 10-7 A/cm, 3 x 10-7 A/cm, and 5 x 10-11 A/cm, respectively. Calculate the current densities and the polarization resistances if the reactions proceed at n=-0.08 V versus SHE. The electrode surface area is 1 cm? and a=0.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


