Question: The experimental data in the table was collected during a freezing point depression study where BHT (butylated hydroxytoluene) was the solvent. Use this data to
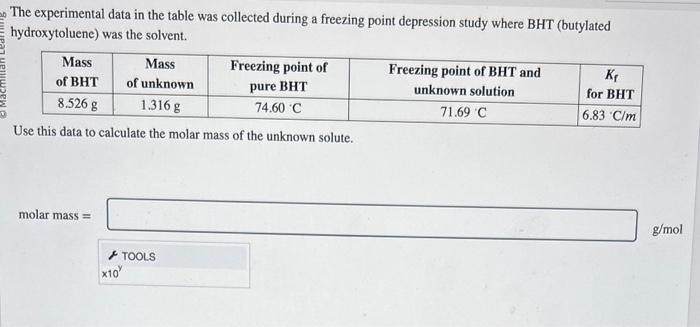
The experimental data in the table was collected during a freezing point depression study where BHT (butylated hydroxytoluene) was the solvent. Use this data to calculate the molar mass of the unknown solute. molar mass = g/mol
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


