Question: The figure below shows the DLVO interaction force versus the separation between two colloidal particles in 1 mM NaCl and 1 M NaCl solution. a)
The figure below shows the DLVO interaction force versus the separation between two colloidal particles in 1 mM NaCl and 1 M NaCl solution.
a) Identify which curve corresponds to 1 mM NaCl and which one to 1 M NaCl. (1 point)
b) At what separations is the interaction between the particles repulsive in curves A and B? (2 points)
c) Explain why the shape of the curves is different at different NaCl concentrations. (2 points)
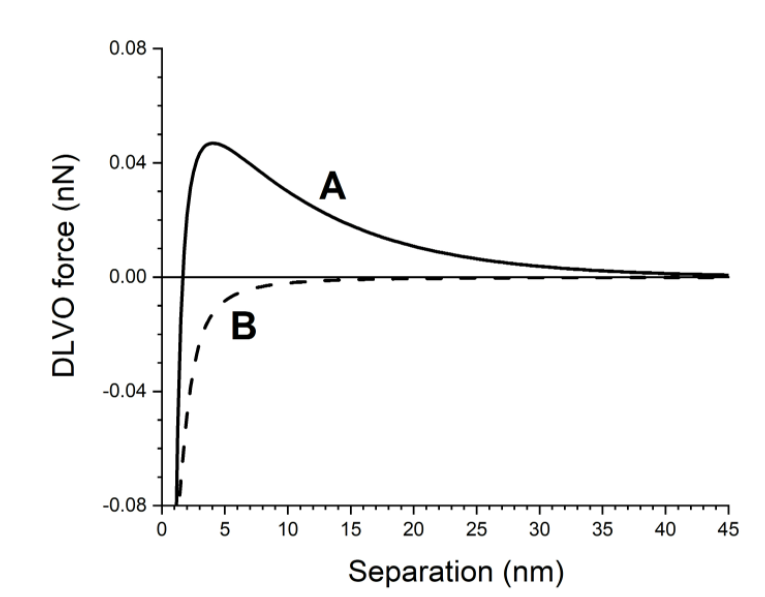
0.08 0.04 A DLVO force (nN) 0.00 B -0.04 -0.08 05 10 15 20 25 30 35 40 45 Separation (nm)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


