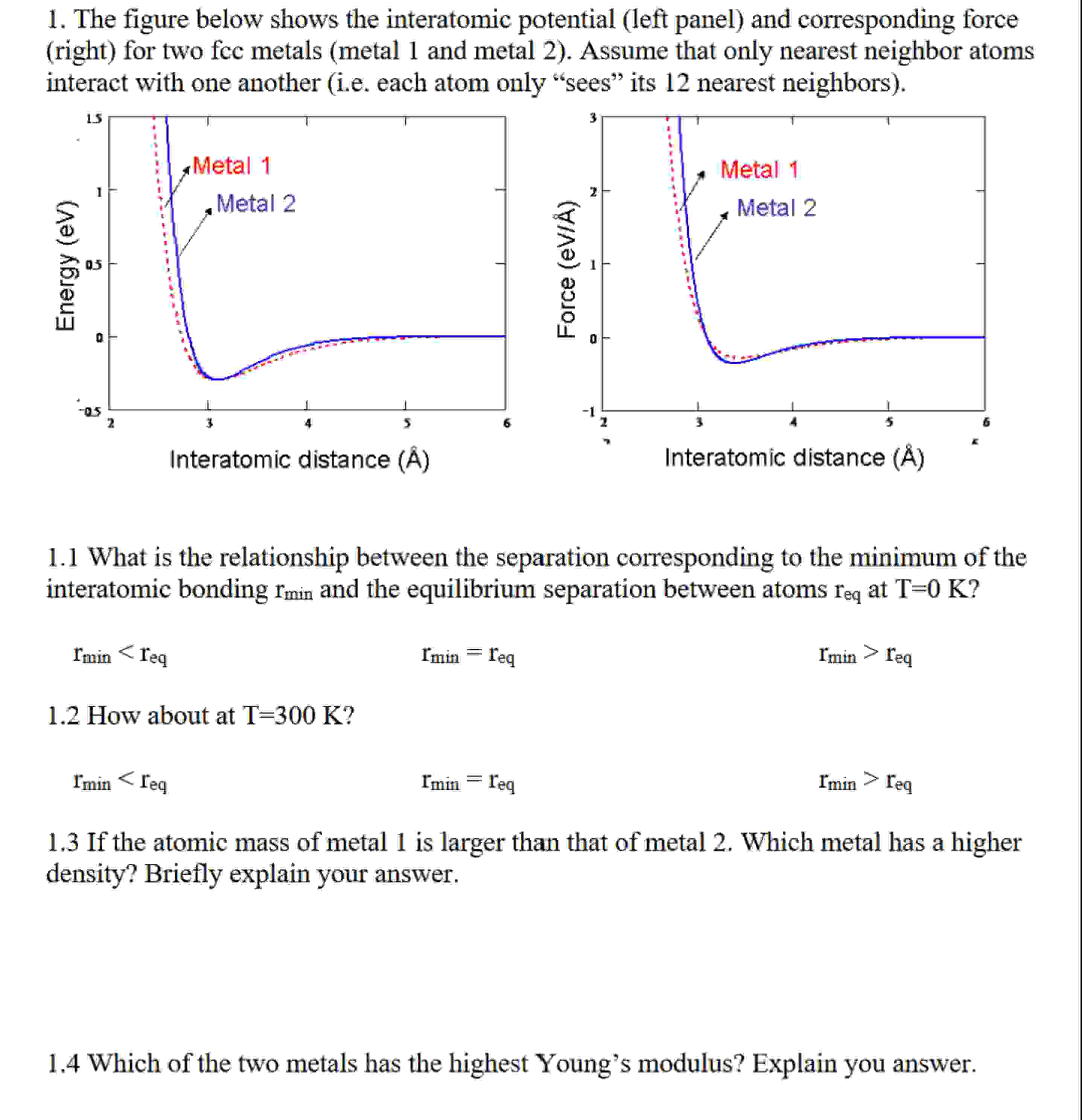
Question: The figure below shows the interatomic potential ( left panel ) and corresponding force ( right ) for two fcc metals ( metal 1 and
The figure below shows the interatomic potential left panel and corresponding force
right for two fcc metals metal and metal Assume that only nearest neighbor atoms
interact with one another ie each atom only "sees" its nearest neighbors
What is the relationship between the separation corresponding to the minimum of the
interatomic bonding and the equilibrium separation between atoms at
the atomic mass metal larger than that metal Which metal has a higher
density? Briefly explain your answer.
Which the two metals has the highest Young's modulus? Explain you answer.
How about
the atomic mass metal larger than that metal Which metal has a higher
density? Briefly explain your answer.
Which the two metals has the highest Young's modulus? Explain you answer.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


