Question: The Figure shows a P-x diagram for the binary system of ethane and n-pentane at 300 K. a.) Is the critical temperature of pure ethane
The Figure shows a P-x diagram for the binary system of ethane and n-pentane at 300 K.
a.) Is the critical temperature of pure ethane lower than 300 K? Explain why, or why not.
b.) 12 gram of ethane is initially in a PVT cell at 300 K and 1500 kPa. Then, a certain amount of n-pentane is added to reach a bubble point at this temperature-pressure condition. How many grams of n-pentane should be injected into the PVT cell? The molecular weight of ethane(30.070g/mol) and n-pentane(72.151 g/mol). The ethane concentration in the equilibrium liquid phase is 43 mol%, and that in the equilibrium vapor phase is 95 mol%, at 300 K and 1500 kPa.
c.) An additional amount of n-pentane ethane is added to the mixture so that the overall ethane concentration in the mixture is 60 mol%. Then, two phases (vapor and liquid) form at equilibrium at 300 K and 1500 kPa. What is the mole fraction of the vapor phase?
d.) The diagram shows a few tie lines. Are these tie lines horizontal in this diagram? Why or why not? Discuss equilibrium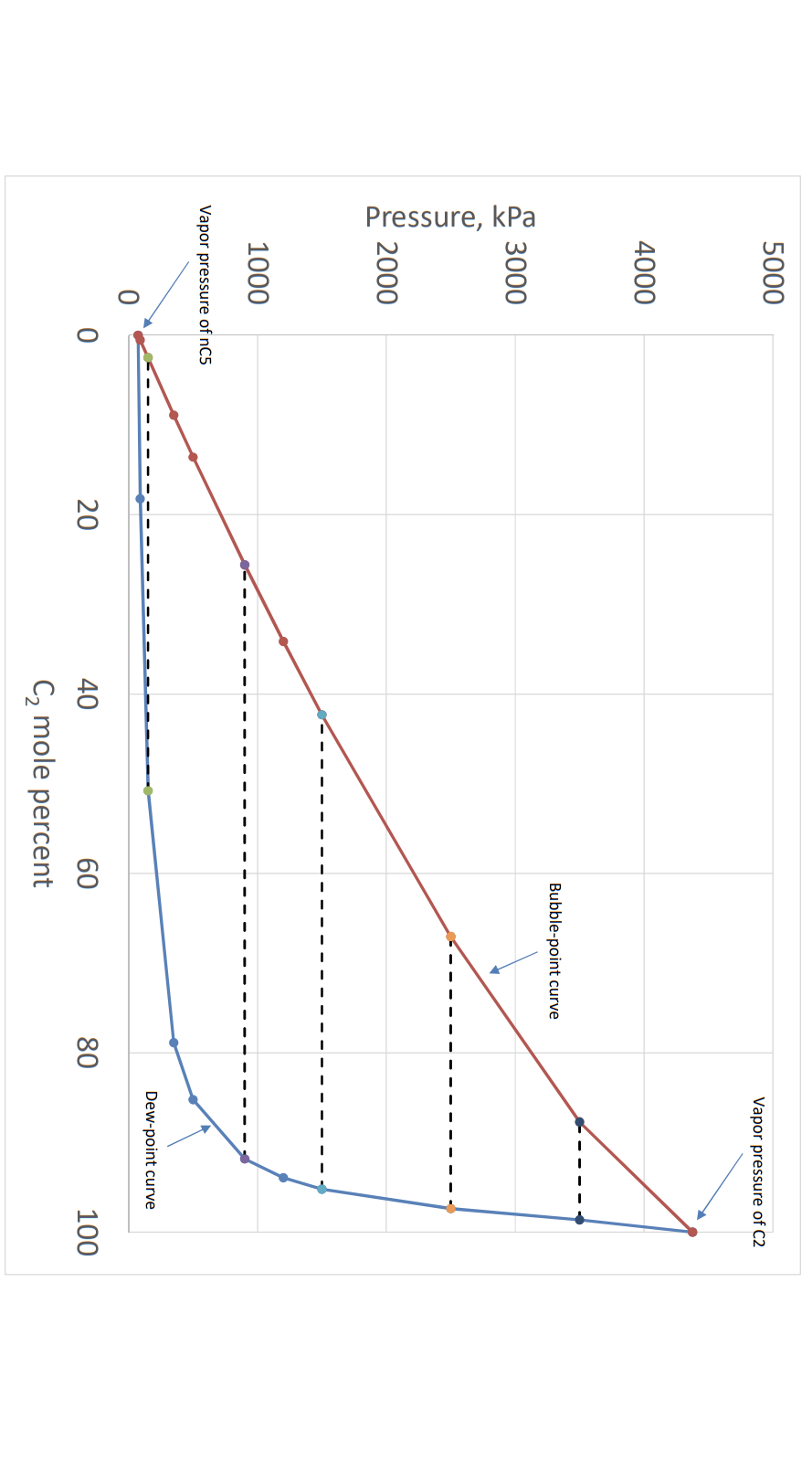
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


