Question: the first pic is the problem, the second one is the figure required, and the 3rd one just in case you need more information, the


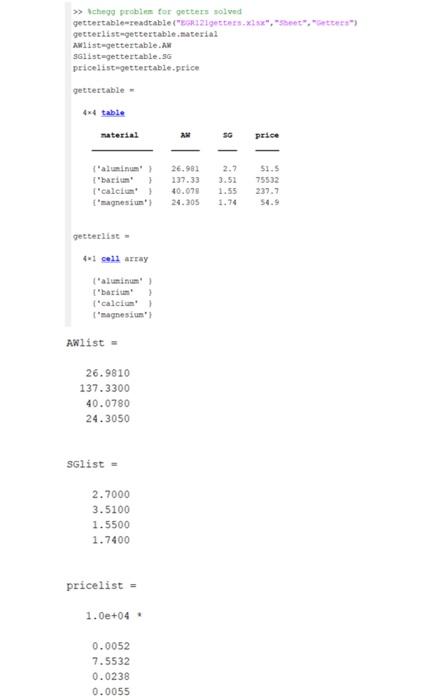
Part 1 - Aluminum getter only (20 pts) In this part we will implement the FIGURE solutions calculations for 1.5 mmol of aluminum. 1. Start a new Section with a title comment. 2. Create variables for all given quantities and assign the given values. ("Info"} Do not define AW and SG. You already have AWlist and SGlist. The values for aluminum are the first values in each vector AWlist(1) and SGlist(1). Create two variables for quantity: millmol and mol. You will need both. For unit conversions, you may create a variable or just use the number. Document units for all variables and conversions in comments. 3. Refer to your FIGURE solution. ("General approach and "Unifying equations"} 4. Compute the thickness of aluminum. ("Run the numbers", aka computation code") Required variable name: x_al Required units: microns You can compute intermediate values! Note ALL units in a comment!! 5. Use fprintf commands to create the following display, where #.# is the correct value, aka ("Report") Coating a 3/4" diameter vacuum tube with 1.5 mmol aluminum results in a #.#-micron thick coating. You must refer to the variable x_al for the thickness value #., and display 3 SF (960.38). 6. Run the section. The answer should be your FIGURE answer! If not, look for errors, starting with unit conversion. Part 1b In this part, we will convert the quantity variable to a vector and create a graph. 1. Change the variable millimol to a row vector, equal to 1.5 to 2.5 mmol, in increments of 0.1 mmol. When you run the code, x_al will now also be a vector, corresponding to the millimol vector. Add the dot operator only as needed. 2. Replace x_al in the fprintf command with x_al(1). Because millimol(1) = 1.5 mmol, x_al(1) should be the same as in Part la. The printed statement should be the same too! 3. Run the section and make sure the printed value is correct. 4. Print the quantity in millimoles and the thickness in microns in two side by side columns. ("Report"} Hint: setT = (millimole x_al), then disp(T). Use a disp or fprintf command to write a line of column titles first. The default number of decimal places is fine. 5. Graph the layer thickness in microns (x_at) vs quantity in millimoles (mmol). {"Report (graphically)"} Use markers. Use good graph presentation, e.g. include title and axis labels with units. kg Mass of Al 1.5 millimaks = 1.5x10-326.981/1000 4.05 X10**kq corresponding Volume = 4,05x10-27 =1.499 X10 m3 it. If thickness isx, then I deposited in hemis Phena! , v Part = 4TX (095 25x10 2 X 0.0011401 y equating I) and (2) X= 1 X= 0.013148 m) or 113.1481 mm NE >> chego problem for getters solved gettortable readtable (E2getters.xlsx", "set, getterlisteget tertable material Alist-gettertable. Solistegettertable.SG pricelist gettertable price getter table- 4 4 table material 23 SG price f'aluminum! > 26.981 137.33 40.070 24.305 2.7 3.52 1.55 1.74 $1.5 75532 239.7 54.9 ('calcium Imagnesium getterlist 41 cell array L'aluminum! ['barium {"calciun magnesium Alista 26.9810 137.3300 40.0780 24.3050 SGlist - 2.7000 3.5100 1.5500 1.7400 pricelist - 1.0e+04 0.0052 7.5532 0.0238 0.0055 Part 1 - Aluminum getter only (20 pts) In this part we will implement the FIGURE solutions calculations for 1.5 mmol of aluminum. 1. Start a new Section with a title comment. 2. Create variables for all given quantities and assign the given values. ("Info"} Do not define AW and SG. You already have AWlist and SGlist. The values for aluminum are the first values in each vector AWlist(1) and SGlist(1). Create two variables for quantity: millmol and mol. You will need both. For unit conversions, you may create a variable or just use the number. Document units for all variables and conversions in comments. 3. Refer to your FIGURE solution. ("General approach and "Unifying equations"} 4. Compute the thickness of aluminum. ("Run the numbers", aka computation code") Required variable name: x_al Required units: microns You can compute intermediate values! Note ALL units in a comment!! 5. Use fprintf commands to create the following display, where #.# is the correct value, aka ("Report") Coating a 3/4" diameter vacuum tube with 1.5 mmol aluminum results in a #.#-micron thick coating. You must refer to the variable x_al for the thickness value #., and display 3 SF (960.38). 6. Run the section. The answer should be your FIGURE answer! If not, look for errors, starting with unit conversion. Part 1b In this part, we will convert the quantity variable to a vector and create a graph. 1. Change the variable millimol to a row vector, equal to 1.5 to 2.5 mmol, in increments of 0.1 mmol. When you run the code, x_al will now also be a vector, corresponding to the millimol vector. Add the dot operator only as needed. 2. Replace x_al in the fprintf command with x_al(1). Because millimol(1) = 1.5 mmol, x_al(1) should be the same as in Part la. The printed statement should be the same too! 3. Run the section and make sure the printed value is correct. 4. Print the quantity in millimoles and the thickness in microns in two side by side columns. ("Report"} Hint: setT = (millimole x_al), then disp(T). Use a disp or fprintf command to write a line of column titles first. The default number of decimal places is fine. 5. Graph the layer thickness in microns (x_at) vs quantity in millimoles (mmol). {"Report (graphically)"} Use markers. Use good graph presentation, e.g. include title and axis labels with units. kg Mass of Al 1.5 millimaks = 1.5x10-326.981/1000 4.05 X10**kq corresponding Volume = 4,05x10-27 =1.499 X10 m3 it. If thickness isx, then I deposited in hemis Phena! , v Part = 4TX (095 25x10 2 X 0.0011401 y equating I) and (2) X= 1 X= 0.013148 m) or 113.1481 mm NE >> chego problem for getters solved gettortable readtable (E2getters.xlsx", "set, getterlisteget tertable material Alist-gettertable. Solistegettertable.SG pricelist gettertable price getter table- 4 4 table material 23 SG price f'aluminum! > 26.981 137.33 40.070 24.305 2.7 3.52 1.55 1.74 $1.5 75532 239.7 54.9 ('calcium Imagnesium getterlist 41 cell array L'aluminum! ['barium {"calciun magnesium Alista 26.9810 137.3300 40.0780 24.3050 SGlist - 2.7000 3.5100 1.5500 1.7400 pricelist - 1.0e+04 0.0052 7.5532 0.0238 0.0055
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


