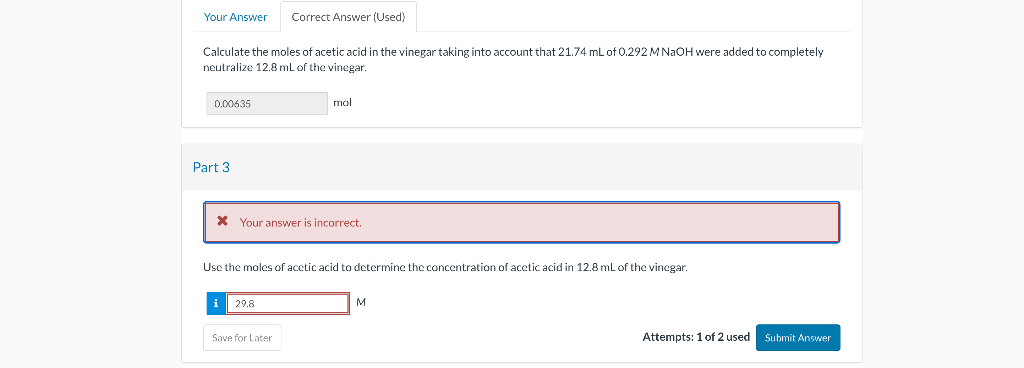
Question: The first question is correct and context for the second question Calculate the moles of acetic acid in the vinegar taking into account that 21.74mL
 The first question is correct and context for the second question
The first question is correct and context for the second question
Calculate the moles of acetic acid in the vinegar taking into account that 21.74mL of 0.292MNaOH were added to completely neutralize 12.8mL of the vinegar. mol art 3 Use the moles of acetic acid to determine the concentration of acetic acid in 12.8mL of the vinegar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


