Question: The flask shown here contains 10.0 mL of HCI and a few drops of phenolphthalein indicator. The buret contains 0.290 M NAH. mL Reset
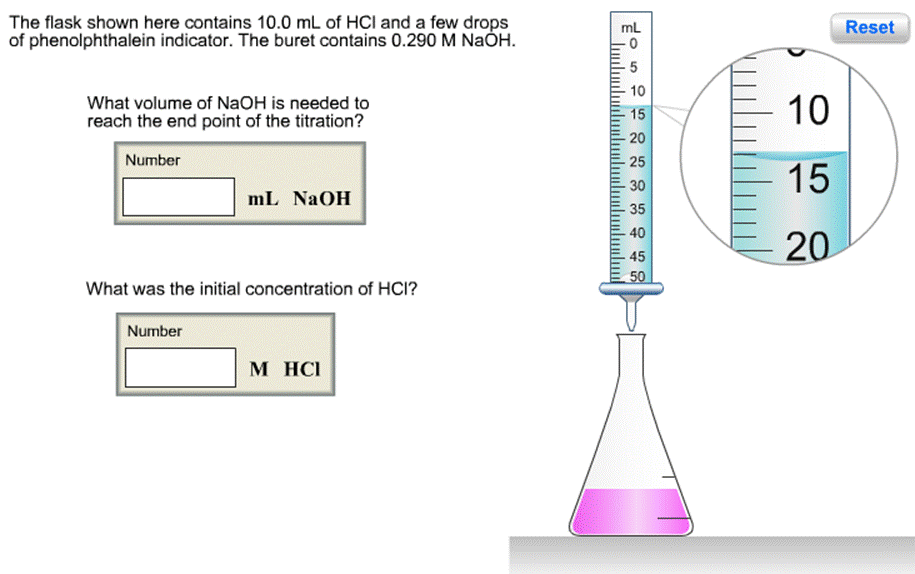
The flask shown here contains 10.0 mL of HCI and a few drops of phenolphthalein indicator. The buret contains 0.290 M NAH. mL Reset 10 What volume of NaOH is needed to reach the end point of the titration? 10 15 20 Number 25 15 30 mL NAOH 35 40 20 45 50 What was the initial concentration of HCI? Number M HCI Click to begin The flask shown here contains 10.0 mL of HCI and a few drops of phenolphthalein indicator. The buret contains 0.290 M NAH. mL Reset 10 What volume of NaOH is needed to reach the end point of the titration? 10 15 20 Number 25 15 30 mL NAOH 35 40 20 45 50 What was the initial concentration of HCI? Number M HCI Click to begin The flask shown here contains 10.0 mL of HCI and a few drops of phenolphthalein indicator. The buret contains 0.290 M NAH. mL Reset 10 What volume of NaOH is needed to reach the end point of the titration? 10 15 20 Number 25 15 30 mL NAOH 35 40 20 45 50 What was the initial concentration of HCI? Number M HCI Click to begin
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
15 griuen thed Velume of Uve lom Volume of Ma on V6 Conc... View full answer

Get step-by-step solutions from verified subject matter experts


