Question: The following basic reaction is carried out in a liquid MFR . 1 mol / m 3 of pure reactant A flows into the reactor
The following basic reaction is carried out in a liquid MFR molm of pure reactant A flows into the reactor at a rate of mmin If one reactor with volume m is used and two reactors with volume m are used in series, find the concentration of R that can be obtained at the outlet of the reactor in each case, compare the results with each other and explain why.
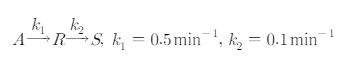
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


