Question: The following data were recorded using a spectrophotometer for a set of standard solutions containing cobalt(II) ions: (a) Construct a Beer's law graph for the
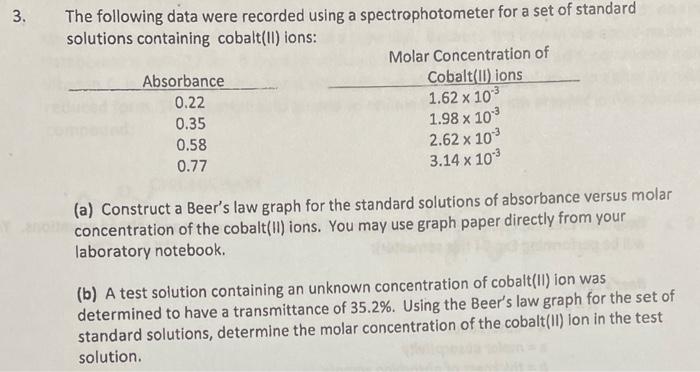


The following data were recorded using a spectrophotometer for a set of standard solutions containing cobalt(II) ions: (a) Construct a Beer's law graph for the standard solutions of absorbance versus molar concentration of the cobalt(II) ions. You may use graph paper directly from your laboratory notebook. (b) A test solution containing an unknown concentration of cobalt(II) ion was determined to have a transmittance of 35.2%. Using the Beer's law graph for the set of standard solutions, determine the molar concentration of the cobalt(II) lon in the test solution. Suppose it was determined in Experiment 398 that 0.610 mol of oxalate ion was present in 100g of the coordination compound. How many milliliters of 0.500MCaCl2 would be needed to precipitate the oxalate ion in a 0.100g sample of the coordination compound? Assume the reaction: Ca2+(aq)+C2O42(aq)CaC2O4(s) Equations (See experiment 34 for details on the spectrophotometer and related equations. You will be performing Exp 34 this semester): Beer's Law A=abcAabc=absorbance=molarabsorptivity=thicknessofabsorbingsample=molarconcentrationofabsorbingspecies Absorbance (A) vs \%Transmittance: %T=10A100% %T=% of light that passes through the sample. log(%T/100%)=A
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


