Question: The following equations may be needed to answer question 2 mm m+m Ej = h B J (J + 1) Ev = hcv (v
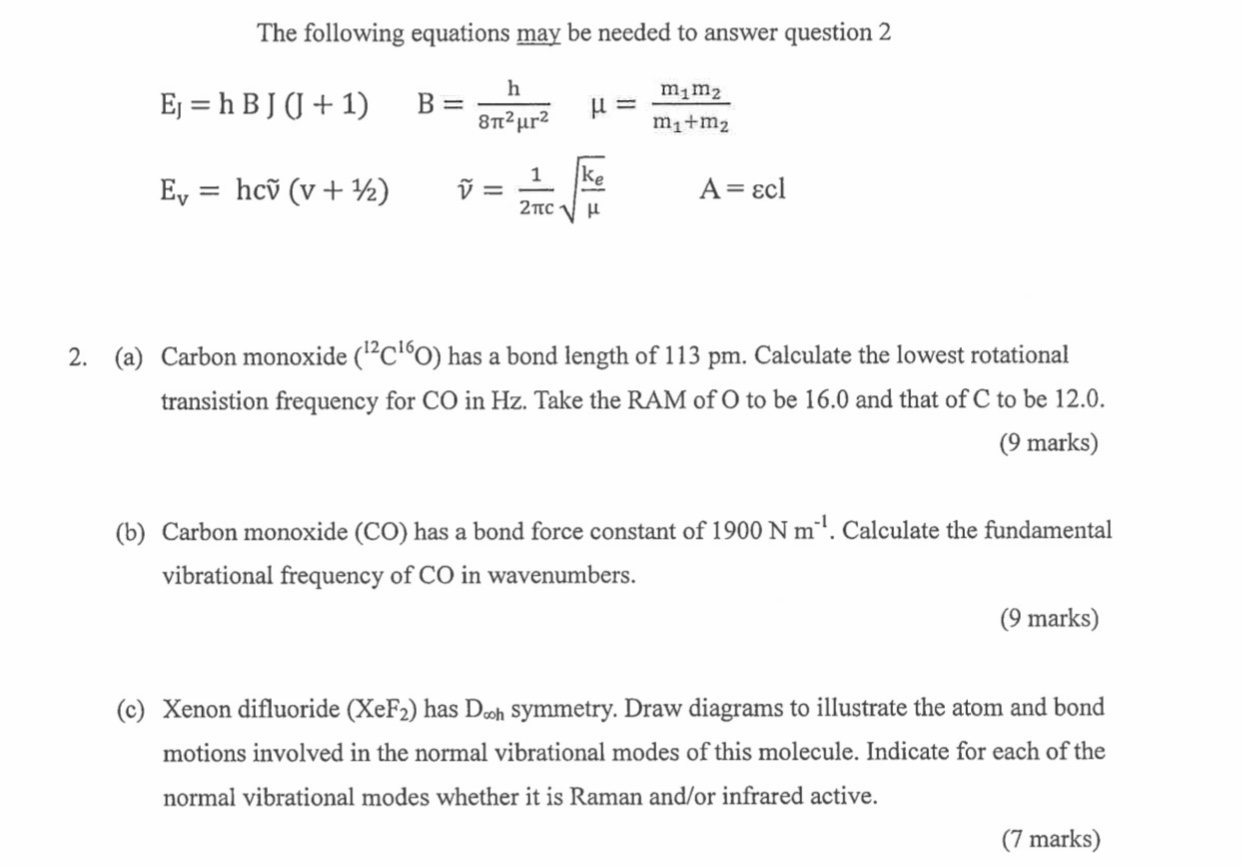
The following equations may be needed to answer question 2 mm m+m Ej = h B J (J + 1) Ev = hcv (v + ) B = h 82ur2 V = = 1 ke 2TCH A = Ecl 2. (a) Carbon monoxide (C60) has a bond length of 113 pm. Calculate the lowest rotational transistion frequency for CO in Hz. Take the RAM of O to be 16.0 and that of C to be 12.0. (9 marks) (b) Carbon monoxide (CO) has a bond force constant of 1900 N m. Calculate the fundamental vibrational frequency of CO in wavenumbers. (9 marks) (c) Xenon difluoride (XeF) has Dooh symmetry. Draw diagrams to illustrate the atom and bond motions involved in the normal vibrational modes of this molecule. Indicate for each of the normal vibrational modes whether it is Raman and/or infrared active. (7 marks)
Step by Step Solution
3.53 Rating (160 Votes )
There are 3 Steps involved in it
Lets go through each part of the question and provide the correct answers a Rotational Transition Frequency of CO Given Bond length of CO r 113 pm 113... View full answer

Get step-by-step solutions from verified subject matter experts


