Question: The following initial rate data are for the reaction for the formation of phosgene from carbon monoxide and chlorine: CO + Cl2 COCI2 M Initial
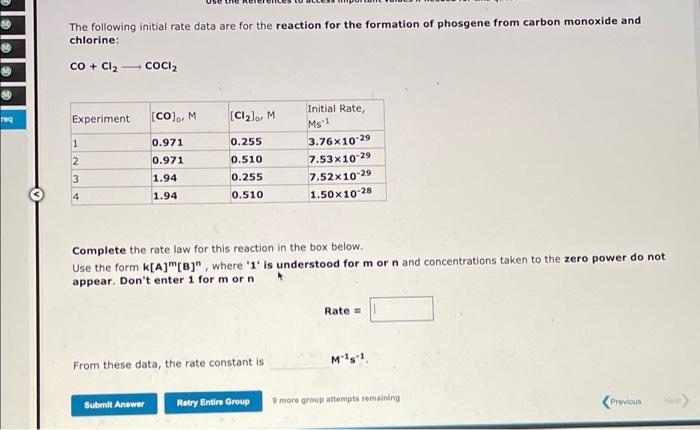
The following initial rate data are for the reaction for the formation of phosgene from carbon monoxide and chlorine: CO + Cl2 COCI2 M Initial Rate, reg Experiment [CO), M [Cllo, M Ms1 1 0.971 0.971 2 0.255 0.510 0.255 0.510 3.76x10-29 7.53x10-29 7.52x10-29 1.50x10-28 3 1.94 1.94 4 Complete the rate law for this reaction in the box below. Use the form k[A]"(B)", where '1' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for morn Rate From these data, the rate constant is M's1 Submit Answer Retry Entire Group more group attempts remaining Previous
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


