Question: The following molecule allows three rotation angles per bond (two bonds). Each bond rotation angle is independent and can exist in 3 states; high energy
The following molecule allows three rotation angles per bond (two bonds). Each bond rotation angle is independent and can exist in 3 states; high energy (2 kl/mol), middle energy (1 kJ/mol), and low energy (0 kJ/mol). Find the probability that one bond is in a middle energy state and the other bond is in a low energy state.
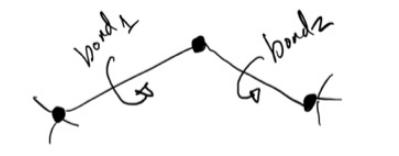
Tprod bondz
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
To solve this problem well follow these steps Step 1 Define the Energy States Each bond can be in on... View full answer

Get step-by-step solutions from verified subject matter experts


