Question: The following rate data is given for a liquid phase reaction AB + C. The reactor type is a batch reactor. Time (mins) 0 10
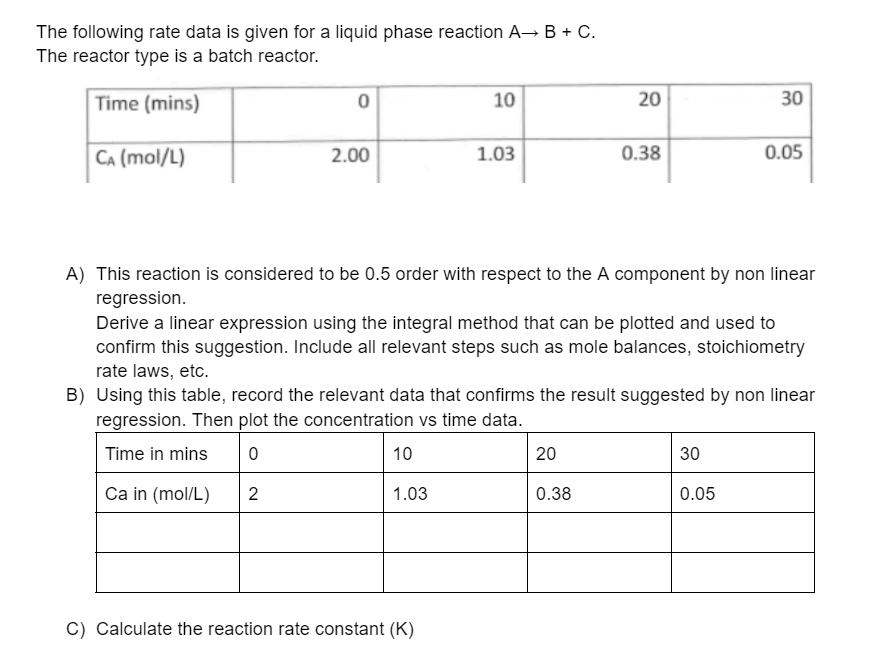
The following rate data is given for a liquid phase reaction AB + C. The reactor type is a batch reactor. Time (mins) 0 10 20 30 CA (mol/L) 2.00 1.03 0.38 0.05 A) This reaction is considered to be 0.5 order with respect to the A component by non linear regression. Derive a linear expression using the integral method that can be plotted and used to confirm this suggestion. Include all relevant steps such as mole balances, stoichiometry rate laws, etc. B) Using this table, record the relevant data that confirms the result suggested by non linear regression. Then plot the concentration vs time data. Time in mins 0 10 20 30 Ca in (mol/L) 2 1.03 0.38 0.05 C) Calculate the reaction rate constant (K)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


