Question: The following reactors in sequence are used to convert methane to hydrogen: (i) Steam methane reforming: CH4+H2O=CO+3H2; endothermic; 750800C (ii) High temperature water gas shift:
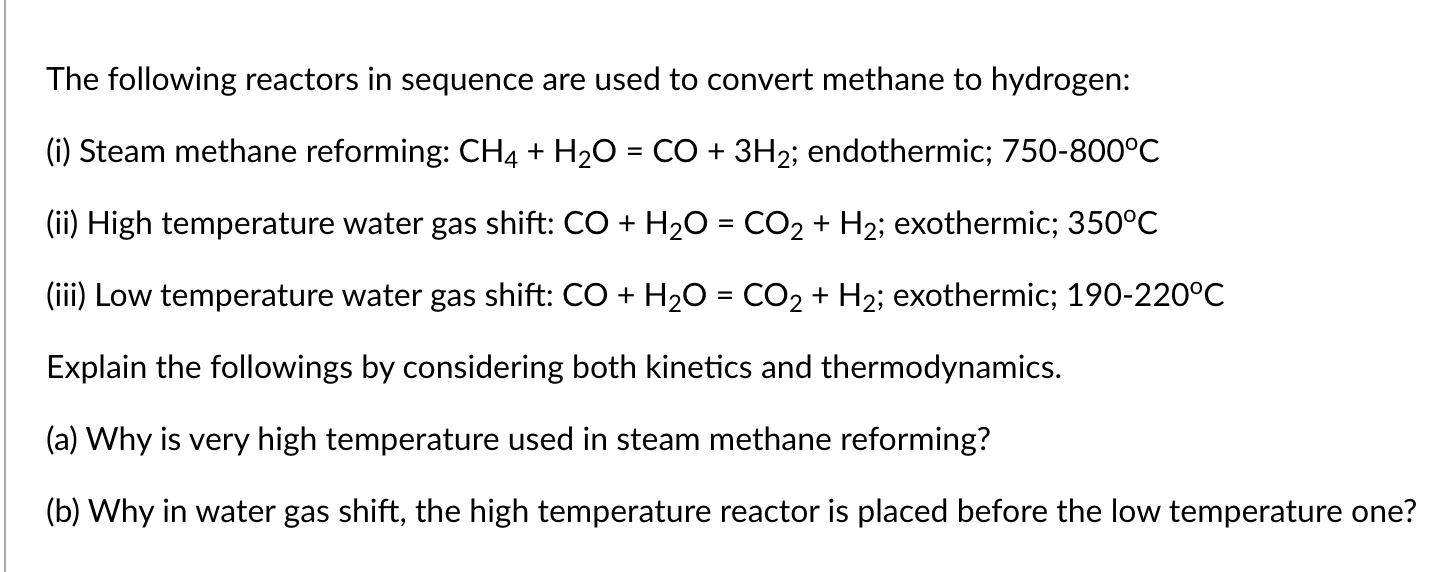
The following reactors in sequence are used to convert methane to hydrogen: (i) Steam methane reforming: CH4+H2O=CO+3H2; endothermic; 750800C (ii) High temperature water gas shift: CO+H2O=CO2+H2; exothermic; 350C (iii) Low temperature water gas shift: CO+H2O=CO2+H2; exothermic; 190-220 C Explain the followings by considering both kinetics and thermodynamics. (a) Why is very high temperature used in steam methane reforming? (b) Why in water gas shift, the high temperature reactor is placed before the low temperature one
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


