The autothermal reforming of methane to hydrogen was described in Example 4.2. The solution in the example
Question:
The autothermal reforming of methane to hydrogen was described in Example 4.2. The solution in the example was not optimized, and suggestions were given for how to improve the results. Optimize the process to minimize the cost of production of hydrogen, assuming:
Data from example 4.2
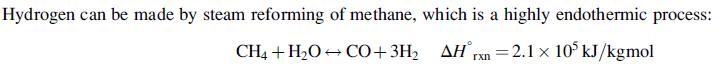
Steam reforming is usually carried out in fired tubular reactors, with catalyst packed inside the tubes and fuel fired on the outside of the tubes to provide the heat of reaction. The product gas mixture contains carbon dioxide and water vapour as well as carbon monoxide and hydrogen and is conventionally known as synthesis gas or syngas. Hydrogen can also be made by partial oxidation of methane, which is an exothermic process, but yields less product per mole of methane feed:
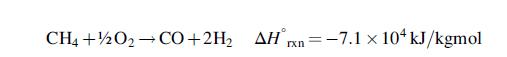
When steam, oxygen and methane are combined, heat from the partial oxidation reaction can be used to provide the heat for steam reforming. The combined process is known as autothermal reforming. Autothermal reforming has the attraction of requiring less capital investment than steam reforming (because it does not need a fired-heater reactor), but giving higher yields than partial oxidation. The yield of hydrogen can be further increased by carrying out the water gas shift reaction:
![]()
The water gas shift reaction equilibrates rapidly at temperatures above about 450 °C. At high temperatures this reaction favours the formation of carbon monoxide, whilst at low temperatures more hydrogen is formed. When hydrogen is the desired product, the shift reaction is promoted at lower temperatures by using an excess of steam and providing a mediumor low-temperature shift catalyst.
In an autothermal reforming process, 1000 kmol/h of methane at 20 °C is compressed to 10 bar, mixed with 2500 kmol/h of saturated steam and reactedwith pure oxygen to give 98%conversion of the methane. The resulting products are cooled and passed over a medium-temperature shift catalyst that gives an outlet composition corresponding to equilibrium at 350 °C.
i). How much heat is required to vaporize the steam?
ii). How much oxygen is needed?
iii). What is the temperature at the exit of the autothermal reforming reactor?
iv). What is the final molar flow rate of each component of the synthesis gas?
1. Cost of methane = 16¢/lb
2. Cost of oxygen = 2¢/lb
3. Cost of water = 25 ¢/1000 lb
4. Annualized cost of heat exchangers = $ 30,000 + 3 A, where A is the area in ft2
5. Cost of electric power ¼ 6 ¢/kWh
6. Reactor and catalyst costs are the same in all cases.
First determine the optimal heat recovery and steam and oxygen to methane ratios for a given methane conversion. Repeat for different methane conversions to find the overall optimum.
Step by Step Answer:

Chemical Engineering Design
ISBN: 9780081025994
6th Edition
Authors: Ray Sinnott, R.K. Sinnott, Sinnott Gavin Towler





