Question: The gas phase reaction: A B + C: -A = KCA k=0.1 min-1 is to be carried out in a CSTR followed by a PFR
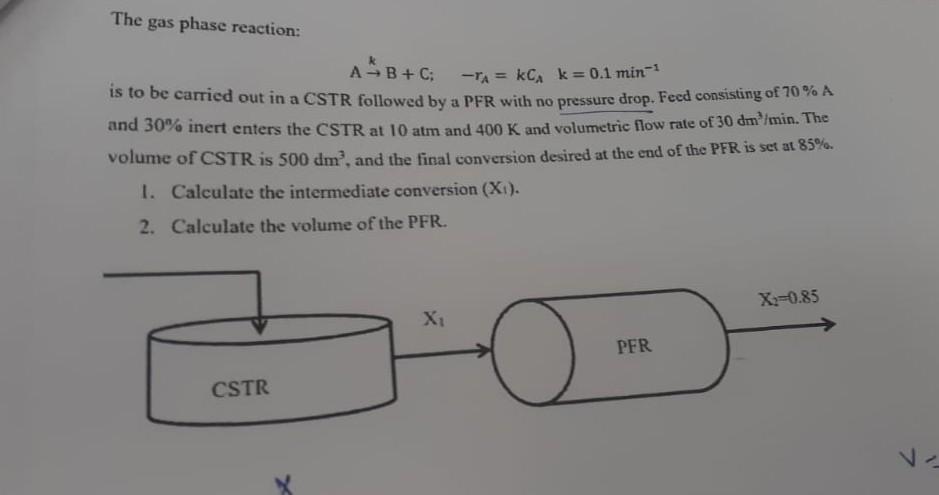
The gas phase reaction: A B + C: -A = KCA k=0.1 min-1 is to be carried out in a CSTR followed by a PFR with no pressure drop. Feed consisting of 70 % A and 30% inert enters the CSTR at 10 atm and 400 K and volumetric flow rate of 30 dm /min. The volume of CSTR is 500 dm, and the final conversion desired at the end of the PFR is set at 85%. 1. Calculate the intermediate conversion (Xi). 2. Calculate the volume of the PFR. X2=0.85 X PFR CSTR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


